This chapter discusses benign and malignant renal lesions ( Box 63-1 ) with a separate note on cystic lesions based on the Bosniak classification.
Benign Lesions
- •
Simple renal cyst
- •
Oncocytoma
- •
Angiomyolipoma
- •
Leiomyoma
- •
Mesoblastic nephroma
- •
Adenoma
Malignant Lesions
- •
Renal parenchymal tumors, including renal cell carcinoma subtypes
- •
Urothelial carcinoma
- •
Secondary renal tumors
- •
Lymphoma and leukemia
- •
- •
Metastatic lesions
- •
Pediatric malignant tumors
- •
Wilms’ tumor
- •
Nephroblastomatosis
- •
Clear cell sarcoma
- •
Rhabdoid tumor
- •
Benign Lesions
The Simple Renal Cyst
Etiology, Prevalence, and Epidemiology
The incidental detection of cystic renal lesions has dramatically increased with the widespread use of ultrasonography and cross-sectional imaging. As many as 27% of patients older than 50 years have a benign renal cyst on computed tomography (CT) examination. Using strict criteria and proper technique, a simple renal cyst can be diagnosed using ultrasonography, CT, and magnetic resonance imaging (MRI).
Imaging
Ultrasonography.
The sonographic criteria for a simple cyst are well established and include (1) anechoic contents, (2) sharp smooth walls, (3) posterior acoustic enhancement, and (4) lack of internal blood flow.
Computed Tomography.
Findings characteristic of a simple renal cyst on CT are (1) water density (−20 to 20 Hounsfield units [HU]) on precontrast images, (2) smooth thin borders with a sharp interface with the adjacent renal parenchyma, and (3) does not enhance with contrast.
Magnetic Resonance Imaging.
A lesion can be categorized as a simple renal cyst on MRI, if the lesion (1) has a signal intensity that follows simple fluid, (2) has smooth thin borders with a sharp interface with the adjacent renal parenchyma, and (3) does not enhance on postcontrast images.
Clinical Implications.
A renal cyst that meets each of the criteria for any given imaging modality can be confidently diagnosed as a benign simple cyst and requires no additional evaluation ( Figure 63-1 ).

Angiomyolipoma
Etiology, Prevalence, and Epidemiology
Angiomyolipoma is a benign renal tumor composed of varying amounts of blood vessels, fat, and smooth muscle. The majority of angiomyolipomas are sporadic (80%) and are typically identified in adults, with a strong female predilection as compared to men with a ratio as high as 4 : 1. A smaller percentage of approximately 20% are associated with tuberous sclerosis complex, and it can occur in as many as 55% 75% of patients with it.
Clinical Presentation
Angiomyolipomas are often an incidental finding on imaging. In a minority of cases, symptoms such as flank pain, nausea, vomiting, and fever are produced by mass effect and intratumoral or perirenal hemorrhage. The risk for bleeding has been reported to be proportional to the size of the lesion once it is larger than 4 cm in diameter.
Pathophysiology
Isolated angiomyolipomas are usually solitary, whereas angiomyolipomas associated with tuberous sclerosis are often multiple, larger, and bilateral. The majority of these tumors are intraparenchymal, but 25% are exophytic.
Pathology
Angiomyolipomas belong to the PEComa family. PEComas are considered a family of perivascular epithelioid cell (PEC) tumors (PEComa) and are essentially mesenchymal neoplasms. Abnormalities of the blood vessels associated with angiomyolipomas explain the propensity for these lesions to hemorrhage.
On gross examination, angiomyolipomas are composed predominantly of fat and have a homogeneous yellow appearance. Tumors with more varied proportions of fat, smooth muscle, and blood vessels have a heterogeneous gross pathologic appearance. Angiomyolipomas are often well circumscribed but lack a true capsule.
Imaging
The imaging appearance of angiomyolipomas varies significantly because of its variable composition and can cause a diverse radiographic appearance of these lesions. However, when a large fat component is present, the imaging characteristics are usually pathognomonic and vice versa.
Radiography.
Intravenous urography and plain radiography are generally not sensitive techniques for identifying these tumors.
Computed Tomography.
The value of CT is in identifying any fat content present in these lesions because the presence of intratumoral fat is nearly pathognomonic of angiomyolipoma ( Figure 63-2 ). Lesions with a component demonstrating an attenuation value of less than 20 Hounsfield units (HU) are typical of angiomyolipoma on unenhanced CT. Typically, the lesion is a well-defined, cortical, heterogeneous mass with a fat component. The presence of hemorrhage may cause the lesion to appear poorly defined. Soft tissue attenuation of the lesion may be due to hemorrhage, smooth muscle, or fibrosis.

Approximately 5% of angiomyolipomas have insufficient fat to be recognized on CT and cannot be distinguished from renal cell carcinoma (RCC). Angiomyolipomas without visible fat appear homogeneous and demonstrate higher attenuation than adjacent normal renal parenchyma ( Figure 63-3 ). These lesions also can demonstrate contrast enhancement because of the relatively larger smooth muscle and vascular components.

Calcification is rarely seen but may be present after hemorrhage. In the presence of significant calcification, the diagnosis of angiomyolipoma should be reconsidered.
CT is useful to evaluate for retroperitoneal hemorrhage that may be associated with angiomyolipomas ( Figure 63-4 ). Blood products may obscure the fat content of the lesion, and in these cases carcinoma cannot be excluded.

Magnetic Resonance Imaging.
MRI can be used to depict intratumoral fat. Variable areas of high signal are seen on T1- and T2-weighted images owing to the fat content. However, high signal intensity on T1-weighted images also can be seen with high protein content or hemorrhage. In these cases, fat suppression techniques are useful. Israel and coworkers showed that angiomyolipomas also can be diagnosed using opposed-phase chemical shift MRI in which an India ink artifact at the mass/kidney interface or within a renal mass is suggestive of the tumor ( Figure 63-5 ). Clear cell RCC also may demonstrate signal loss on opposed-phase imaging; therefore, this is not a specific finding for angiomyolipoma.

Ultrasonography.
The typical ultrasound appearance of an angiomyolipoma is a well-defined hyperechoic mass without acoustic shadowing ( Figure 63-6 ). This echogenicity is not necessarily due to the fat component because some lesions that contain little or no fat also can be echogenic. Although RCC can appear echogenic, angiomyolipomas are more likely to show posterior shadowing. These differences, however, cannot distinguish between angiomyolipomas and carcinoma with the same degree of confidence as CT.

Nuclear Medicine.
Nuclear medicine has a limited role in the assessment of angiomyolipoma. When surgical management is indicated, it can be used to assess differential renal function.
Angiography.
Angiography characteristically demonstrates clusters of saccular microaneurysms or macroaneurysms. Other findings on angiography include hypervascularity, venous pooling with a whorled appearance, and lack of arteriovenous shunting ( Figure 63-7 ).

Imaging Algorithm.
Thin-section noncontrast CT is the preferred and most widely available method for detection of angiomyolipoma because demonstration of fat is pathognomonic. Although MRI can detect fat within an angiomyolipoma, it is not as sensitive as CT for detecting fat in small lesions. Opposed-phase chemical shift MRI has been investigated and found to be useful as a method for detecting angiomyolipomas ( Table 63-1 ).
- •
Fat-containing lesion on CT and MRI
- •
Well-circumscribed echogenic mass with posterior shadowing evident on ultrasonography
- •
Retroperitoneal hemorrhage associated with a renal lesion (usually >4 cm)
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Radiography | Poor | Limited sensitivity unless tumor is large and is predominantly fat, which may be seen as radiolucency | Exophytic mass (25%) may not be seen as a space-occupying lesion in the renal sinus |
| CT | High | Minimal fat angiomyolipomas Hemorrhage may mask intratumoral fat | Minimal fat angiomyolipomas Rarely, other benign and malignant lesions may contain fat |
| MRI | High | Failure to detect small lesions or small amount of intratumoral fat because of volume-averaging artifacts and limitations of spatial resolution | Same as for CT Clear cell renal cell carcinoma may demonstrate signal loss on opposed-phase imaging |
| Ultrasonography | Cannot assess for actual presence of fat because hyperechogenicity is not necessarily caused by fat | Echogenic renal cell carcinomas |
Differential Diagnosis
Rare lesions that can contain fat include perirenal fat entrapment or fat necrosis, which can occur in RCC. Other rare entities include myolipoma, liposarcoma, lipoma, oncocytoma, and Wilms’ tumor. In the absence of intratumoral fat, the differential diagnosis includes RCC, leiomyosarcoma, and malignant epithelioid angiomyolipoma.
Treatment
When lesions are small, asymptomatic, and discovered incidentally, no treatment is necessary. Larger lesions if considered at risk for hemorrhage can be prophylactically embolized. In severe acute presentation, total nephrectomy may be required if conservative measures fail.
- •
Most commonly this is an incidental diagnosis in asymptomatic patients.
- •
Lesions greater than 4 cm have increased risk for hemorrhage.
- •
These tumors are often multiple and bilateral in patients with tuberous sclerosis.
Oncocytoma
Etiology, Prevalence, and Epidemiology
Oncocytomas arise from the distal tubule or collecting ducts of the kidney. An oncocyte is a large transformed epithelial cell with a finely granular eosinophilic cytoplasm. These cells increase in number with age and are seen in many organs.
Oncocytomas comprise 3% to 7% of all renal neoplasms. Patients typically present in their sixth to seventh decades. The tumors are more common in males than females in a ratio of 2 : 1.
Clinical Presentation
As many as 75% of oncocytomas are asymptomatic and incidentally diagnosed, but patients may uncommonly present with a flank mass, pain, or hematuria.
Pathophysiology
Most oncocytomas are solitary. Approximately 3% are bilateral and 5% are multicentric within the same kidney. They average 7 cm in diameter and are most often symptomatic when larger than 5 cm.
Pathology
These tumors are well encapsulated and classically have a central scar. On gross inspection, they are tan-brown similar to the renal cortex and well defined. Necrosis, hemorrhage, and calcification are rare.
Imaging
Radiography.
Plain radiographic findings are nonspecific and include a soft tissue mass distorting the renal silhouette with displacement of the fat planes. Calcification is an uncommon feature.
Angiography.
The classic findings are a spokewheel arrangement of vessels, homogeneous dense tumor blush during the capillary phase, and sharp demarcation from the kidney. Bizarre neoplastic vessels are absent, in contrast to RCC.
Computed Tomography.
An oncocytoma typically appears as a well-defined solid renal mass with smooth margins. A central stellate scar is seen in one third of cases, but this is a nonspecific finding that cannot be differentiated from central necrosis in RCC.
On noncontrast CT, oncocytoma is isodense or hyperdense relative to normal renal parenchyma. On the nephrographic phase of contrast-enhanced CT, this tumor is most commonly hypodense to renal parenchyma ( Figure 63-8 ).

Magnetic Resonance Imaging.
Typically, oncocytoma demonstrates low signal on T1-weighted images and high signal on T2-weighted images. The central scar, if present, shows as a low T1 and a low T2 signal, in contrast to necrosis in RCC, which usually has a low T1 and a high T2 signal.
After administration of a contrast agent, oncocytoma typically demonstrates homogeneous enhancement. The central scar generally does not enhance ( Figure 63-9 ).

Ultrasonography.
On ultrasonography, an oncocytoma shows as a well-defined hypoechoic to isoechoic mass ( Figure 63-10 ). If visualized, the central scar may appear echogenic. Doppler imaging may show central radiating vessels in a spokewheel distribution.

Nuclear Medicine.
Nuclear medicine is not routinely used in the evaluation of renal tumors.
Positron Emission Tomography With Computed Tomography.
PET/CT is not routinely performed for evaluation of oncocytoma. It may show equal to slightly higher uptake of fluorodeoxyglucose (FDG) than normal renal parenchyma.
Imaging Algorithm.
Contrast-enhanced CT is the examination of choice for evaluating solid renal masses. Evaluation for vascular involvement, lymphadenopathy, and extent of the lesion is possible ( Table 63-2 ).
- •
Central scar on CT and MRI
- •
Spokewheel pattern of vessels on angiography and Doppler imaging
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Radiography | Poor sensitivity | Cannot distinguish from other space-occupying renal masses | |
| CT | Sensitive but not specific | Cannot distinguish from renal cell carcinoma | Central necrosis of renal cell carcinoma can mimic central scar of oncocytoma. |
| MRI | Sensitive but not specific | Cannot distinguish from renal cell carcinoma | Central scar is not specific for oncocytoma. |
| Ultrasonography | Poor sensitivity | Small isoechoic lesions may be missed. Larger lesions cannot be distinguished from other renal masses. | |
| Nuclear medicine | Poor sensitivity | ||
| PET/CT | Poor sensitivity |
Differential Diagnosis
The main differential diagnostic consideration is RCC. Although central scar on CT/MRI and central radiating vessels on angiography and Doppler imaging are suggestive of oncocytoma, RCC cannot be excluded and histopathologic diagnosis is necessary ( Figure 63-11 ).

Treatment
If radiographic findings suggest the possibility of oncocytoma, percutaneous biopsy may be considered. It is controversial whether oncocytoma can be reliably differentiated from oncocytic RCC on biopsy. Consequently, many clinicians choose pathologic sampling with partial or total nephrectomy. Many oncocytic RCCs are of low metastatic potential and include granular cell carcinoma, chromophobe RCC, and the eosinophilic variant of papillary RCC. Notably, oncocytoma cannot always be reliably distinguished from oncocytic RCC on pathologic examination.
- •
Statistically, RCC is more common than oncocytoma.
- •
Because of overlap in imaging and pathologic findings between oncocytoma and RCC, preoperative diagnosis remains difficult.
- •
Whether oncocytoma can be reliably diagnosed on biopsy is controversial.
Mesoblastic Nephroma
Etiology, Prevalence, and Epidemiology
Mesoblastic nephroma is usually present at birth. It also has been called congenital Wilms’ tumor or fetal mesenchymal hamartoma.
This mesenchymal renal tumor is typically benign but may demonstrate aggressive features such as local invasion or recurrence. There is a male predominance with a peak age at presentation of 3 months. Mesoblastic nephroma is the most common benign fetal renal neoplasm.
Clinical Presentation
The most common manifestation is a large, palpable abdominal mass in a neonate. Less common signs and symptoms include hematuria, hypertension, vomiting, and hypercalcemia. Prenatal hydrops and polyhydramnios also may be seen.
Pathology
On gross examination this tumor has a homogeneous rubbery appearance. The cut surface has a whorled appearance similar to that of uterine leiomyoma. Hemorrhage and necrosis are uncommon. Histologically, there are sheets of fibromatous cells. There is an aggressive variant that is highly cellular with immature mesenchymal cells and a high number of active mitotic figures. This variant has a poorer prognosis and is usually seen in infants and children older than 3 months of age.
Imaging
Radiography.
A large mass may be seen involving the entire kidney. Calcification is uncommon, and radiography is not a reliable modality for evaluating for the presence of renal tumors.
Computed Tomography.
A solid, homogeneous mass arising from the kidney that may replace all or part of the involved kidney. There can be areas of necrosis, although this is uncommon. The tumor does not enhance after contrast agent administration. However, entrapment of nephrons within the mass may cause excretion of contrast agent within the mass. No calcification is evident.
Magnetic Resonance Imaging.
There is low signal intensity on T1-weighted imaging, and the tumor is typically nonenhancing.
Ultrasonography.
A well-circumscribed, homogeneous and hyperechoic mass is evident. Concentric hypoechoic and hyperechoic rings are a helpful imaging feature.
Imaging Algorithm.
Prenatal or pediatric ultrasonography is usually the first imaging study performed when the abdominal mass is palpated. Ultrasonography is easily and widely available; it is inexpensive and involves no ionizing radiation. CT has an important role in evaluating recurrent or metastatic disease.
- •
This homogeneous solid renal mass affects neonates.
- •
The cut surface resembles that of uterine leiomyoma.
Differential Diagnosis
The differential diagnosis includes hydronephrosis and multicystic dysplastic kidney, the most common causes of unilateral renal enlargement in a neonate, and also Wilms’ tumor (different peak age).
Treatment
Patients with the aggressive variant may benefit from adjunctive chemotherapy or irradiation. Surgical resection is the treatment of choice and is curative.
Juxtaglomerular Tumors
Etiology
Juxtaglomerular tumors are renin-producing tumors of juxtaglomerular cells.
Prevalence and Epidemiology
First described by Robertson in 1967, these tumors are also called reninomas. There is a female predominance, and patients are younger than those with essential hypertension.
Clinical Presentation
Patients can present with hypertension with headache, polydipsia, and polyuria. Hypokalemia is due to secondary aldosteronism. Occasionally, acute flank pain, hypotension, and anemia secondary to tumor hemorrhage are noted.
Pathophysiology
These tumors are solitary and 3 to 7 cm in diameter. Most are located beneath the renal capsule, although they may arise near the renal pelvis. Rarely, they may arise from perinephric tissue, possibly from embryonic rests.
Pathology
On gross examination they appear tan or gray. They are sharply defined with a pseudocapsule and commonly contain foci of hemorrhage within them. There is an abundance of small vascular channels.
Imaging
Radiography.
Lack of calcification makes these tumors difficult to identify on plain radiographs.
Computed Tomography.
Juxtaglomerular tumors are small and isodense to renal parenchyma on unenhanced CT. Therefore, contrast-enhanced CT should be performed. Despite numerous small vascular channels, these are hypovascular tumors.
Ultrasonography.
On ultrasonography, an echogenic mass is evident.
Differential Diagnosis
The differential diagnosis includes renal artery stenosis, essential hypertension, and other renin-secreting tumors. One should also consider tumors compressing the renal artery or renal parenchyma (as a cause of hypertension) and RCC and Wilms’ tumor.
Treatment
Surgical excision is curative with subsequent relief of hypertension.
- •
This tumor is a rare, curable cause of hypertension in females.
Adenoma
Etiology
Renal adenomas develop in the proximal convoluted tubule. The cause is unknown.
Prevalence and Epidemiology
At autopsy, 7% to 22% of patients are found to have renal adenoma. They occur in the same age group as RCC, and there is a male predominance.
Clinical Presentation
An adenoma is usually asymptomatic.
Pathophysiology
Adenomas are small, well-differentiated tumors of the renal cortex.
Pathology
Histologically, an adenoma appears similar to low-grade RCC.
Imaging
Adenoma is indistinguishable from RCC on imaging. Most of these lesions are identified at autopsy and are therefore of little clinical significance.
Differential Diagnosis
Adenoma should be differentiated from RCC.
Treatment
Because many authors think that adenoma is an early RCC that has not yet metastasized, these should be treated as such.
Malignant Tumors
Malignant Renal Parenchymal Tumors, Including Renal Cell Carcinoma and Its Subtypes
RCC accounts for approximately 3% of all malignancies and about 3% of cancer deaths in the United States. It is by far the most common renal malignancy, accounting for 85% to 90% of cases.
Etiology
The vast majority of RCCs are sporadic. Environmental risk factors include smoking; unopposed estrogen exposure; obesity (particularly in women); occupational exposure to petroleum products, heavy metals, and asbestos; and hypertension and its treatment.
Patients with acquired cystic kidney disease from chronic hemodialysis are also at increased risk. Hereditary risk factors include von Hippel-Lindau disease, tuberous sclerosis, uterine leiomyosarcoma/RCC syndrome, Birt-Hogg-Dubé syndrome, and familial clear cell carcinoma.
Prevalence and Epidemiology
RCC typically occurs in people older than the age of 40 with a median age at diagnosis in the mid-60s. It is nearly twice as common in men as in women.
Bilateral or multifocal tumors are seen in approximately 5% of sporadic cases, typically with identical histologic subtype in the multiple tumors.
Renal medullary carcinoma is a rare aggressive subtype of RCC, occurring between 10 and 40 years of age (mean age, 22 years) in patients with sickle cell trait.
Clinical Presentation
The classic presenting symptoms include hematuria, flank pain, and flank mass. Many tumors are detected incidentally on imaging. Less commonly, patients present with symptoms of metastatic disease, such as bone pain. Patients also may present with systemic symptoms of malignancy such as fatigue, weight loss, and fever. A well-described presentation in men is new unilateral (usually left) varicocele secondary to compression or obstruction of the ipsilateral renal vein by tumor or thrombus. RCCs can secrete a variety of hormones, including renin, erythropoietin, parathyroid hormone, prolactin, gonadotropin, or adrenocorticotropic hormone, resulting in a paraneoplastic syndrome. Because of the increased use of cross-sectional imaging, up to 60% of RCCs are now detected incidentally.
Pathophysiology
RCCs arise from the renal tubular epithelium of the renal cortex.
Pathology
Grossly, RCCs are expansile, spherical masses. They are usually well marginated and distort the normal renal contour. Rarely, RCC may show an infiltrative growth pattern more typical of urothelial neoplasms.
RCCs are classified by the Heidelberg system, which describes common, conventional, or clear cell RCC (75%), papillary or chromophilic RCC (10%), chromophobe RCC (5%), collecting duct (Bellini duct) carcinoma (1%), and rare unclassified tumors. Medullary carcinoma of the kidney is a rare aggressive subtype of collecting duct carcinoma with an infiltrative growth pattern originally described in young patients with sickle cell trait.
These subtypes carry important prognostic implications. Clear cell RCC accounts for 90% of cases with metastatic disease. Papillary RCC has less metastatic potential, and the chromophobe subtype has the least metastatic potential and best prognosis. The 5-year survival of papillary and chromophobe subtypes (80% to 90%) is significantly higher than for clear cell subtype (50% to 60%). Papillary carcinomas are often multifocal and bilateral, typically presenting at a small size (~3 cm).
Renal medullary carcinoma is extremely aggressive, and metastases, especially to regional lymph nodes, are commonly present at diagnosis. The mean survival after surgery in these patients is only 15 weeks.
RCC is staged using the tumor, node, metastasis (TNM) classification of the American Joint Committee on Cancer or the Robson staging criteria ( Table 63-3 ). Survival is highly correlated with stage. RCC most commonly metastasizes to lung, bone, liver, and brain.
| Stage | Extent of Disease |
|---|---|
| I | Confined to renal capsule |
| II | Extending through renal capsule but confined to Gerota’s fascia |
| IIIa | Regional lymph node involvement |
| IIIb | Renal vein or inferior vena cava extension |
| IIIc | Regional lymph node involvement and extension into renal vein or inferior vena cava |
| IVa | Direct extension beyond Gerota’s fascia |
| IVb | Distant metastases |
Imaging
Radiography.
Conventional abdominal radiography and excretory urography play little role in the modern imaging of RCC. A large renal tumor, particularly one containing calcification, may be detected incidentally on abdominal radiography as an expansile mass ( Figure 63-12 ). Peripheral rim calcification is due to benign cysts in 80% of cases and to RCCs in 20% of cases.
Skeletal plain film imaging may show RCC metastases, which are typically lytic, expansile “bubbly” lesions.
A chest radiograph is usually sufficient in the initial staging of RCC if the primary tumor is small (<3 cm); however, a chest CT should be performed to exclude metastases if the primary tumor is large. In many cases, dedicated CT of the thorax is performed to complete the staging.
Computed Tomography.
Unenhanced and dynamic contrast-enhanced CT scanning of the kidneys with thin-section images are the studies of choice for evaluation of hematuria or suspected renal mass ( Table 63-4 ).
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Radiography | Poor (calcification present in 20% of cases) | Insensitive, nonspecific Requires ionizing radiation | Only detects large renal masses that distort the renal contour |
| Excretory urography | Poor (detects 50% of masses between 2 and 3 cm) | Insensitive, nonspecific Requires ionizing radiation and intravenous contrast | Only detects large renal masses that distort the renal contour |
| Angiography | Specific data are not available to specify accuracy | Invasive, nonspecific Requires ionizing radiation and intravenous contrast | Only detects large renal masses that have visible neovascularity |
| CT | >95% sensitive detection, >90% accurate staging | Requires ionizing radiation and intravenous contrast | Difficult to determine perinephric tumor spread Pseudoenhancement of renal cysts may mimic solid masses Cannot differentiate renal cell carcinoma from benign oncocytoma or “minimal fat” angiomyolipoma |
| MRI | >95% sensitive, 90% accurate staging Often best at depicting stage accurately | Expensive Lack of availability Requires intravenous gadolinium | Calcification not well seen |
| Ultrasonography | Useful to diagnose simple cysts and to differentiate cystic versus solid lesions 79% sensitive for masses <3 cm | Limited detection in obese patients Operator dependent | Small lesions may not be detected |
| Nuclear medicine | Specific data are not available to specify accuracy. | Poor spatial resolution Requires ionizing radiation | |
| PET/CT | Variable (60% to 94% sensitive) | Requires ionizing radiation Expensive Lack of availability | Urinary excretion of fluorodeoxyglucose may obscure a primary renal tumor |
Precontrast images are required to detect renal mass calcification and provide a baseline density measurement to evaluate the degree and pattern of lesion enhancement. Corticomedullary phase images are best for evaluation of lesion vascularity, assessment of vascular invasion, and depiction of renal vascular anatomy for surgical planning. Some lesions, especially smaller lesions or papillary subtype, may be difficult to differentiate from normal renal medulla on corticomedullary phase images. Nephrographic phase images are most sensitive in detection of small lesions. Excretory phase images provide information regarding involvement of the renal collecting system.
On CT, malignant renal parenchymal tumors have a variable appearance. RCC is typically ball shaped and distorts the normal renal shape and contour ( Figure 63-13 ). The majority of lesions are solid (attenuation value >20 HU) and show significant enhancement (~15 HU), with 20% of RCCs appearing at least partially cystic ( Figure 63-14 ).
The CT appearance of RCC varies with lesion size and histologic subtype. Large RCCs, most of which are clear cell type, are often heterogeneous. Papillary RCC is typically hypovascular and homogeneous. Chromophobe RCC may show the spokewheel pattern of contrast enhancement classically associated with oncocytoma. Collecting duct carcinoma and renal medullary carcinoma appear infiltrative and heterogeneous because intratumoral hemorrhage and necrosis.
RCC may invade the renal vein, with tumor thrombus sometimes extending into the inferior vena cava. Thrombus in the renal vein in a patient with RCC may represent direct tumor extension or bland thrombus. These entities can sometimes be differentiated by the presence of thrombus enhancement, which denotes tumor thrombus ( Figure 63-15 ).
CT is more than 95% accurate in the detection of RCC. Preoperative CT is more than 90% accurate in staging RCC, with most errors made in the identification of perinephric tumor spread, which distinguishes T2 from T3a lesions and is difficult to detect on CT because perinephric stranding is nonspecific. The presence of a hypodense, T2-hyperintense pseudocapsule of compressed normal renal parenchyma and fibrous tissue, seen in 66% of cases, is helpful in determining whether the tumor is confined by the renal capsule.
Magnetic Resonance Imaging.
Although CT is the most commonly used imaging modality in the initial evaluation of the upper urinary tract for hematuria or the characterization of a renal mass, MRI is at least as sensitive for the detection and characterization of renal masses owing to its excellent intrinsic soft tissue contrast. MRI accuracy in the staging of RCC is comparable or superior to that of CT.
RCCs typically are isointense or hypointense to renal parenchyma on T1-weighted images, although they may show some T1 hyperintensity because of proteinaceous material or hemorrhage. RCCs usually demonstrate T2 hyperintensity of variable degree, again depending on the size of a cystic or necrotic component. Papillary RCC and collecting duct carcinoma commonly show T2 hypointensity, and chromophobe RCC may show T2 hypointensity.
Dynamic gadolinium-enhanced images typically show a hypervascular tumor ( Figure 63-16 ). The presence of enhancement is required to confidently diagnose a solid renal tumor. Both quantitative and qualitative assessments of enhancement on subtraction images have been shown to be accurate.
Ultrasonography.
Most RCCs are solid, with variable echogenicity. Small RCCs (<3 cm) are more likely to be hyperechoic, which can lead to misdiagnosis as angiomyolipoma. Despite their large size, chromophobe RCCs are homogeneously hyperechoic on ultrasonography ( Figure 63-17 ).
Nuclear Medicine.
Nuclear scintigraphy is not typically used in the initial evaluation of a renal mass. Because 85% of skeletal metastases from RCC are symptomatic, bone scintigraphy is not routinely performed in the initial staging. Moreover, because most RCC metastases have little osteoblastic activity, many do not demonstrate radiotracer uptake on bone scintigraphy.
Positron Emission Tomography With Computed Tomography.
The role of FDG-labeled PET in the detection and evaluation of renal masses has not been fully defined. Normal urinary excretion of FDG may decrease the contrast between FDG-avid tumor and adjacent normal kidney and collecting system, limiting the usefulness of PET in primary tumor detection. Benign renal lesions, such as oncocytoma and inflammation, have been reported to show FDG uptake. In addition, it has been reported that patients with markedly FDG-avid metastases often do not demonstrate FDG avidity of the primary lesion. Thus, FDG-PET may play a role in evaluating distant metastases, in differentiating local tumor recurrence from posttreatment changes, and when conventional imaging of the primary tumor is equivocal.
Differential Diagnosis
The differential diagnosis of RCC depends on its manifestation. A solid renal mass may represent RCC, oncocytoma, lipid-poor angiomyolipoma, hyperdense renal cyst, focal pyelonephritis, metastasis, or lymphoma. A cystic renal lesion may be an RCC, multilocular cystic nephroma, metastasis, cyst complicated by hemorrhage, or focal infectious or inflammatory lesion (including focal pyelonephritis, abscess, or xanthogranulomatous pyelonephritis). The differential diagnosis of an infiltrative renal lesion includes urothelial neoplasm (transitional cell carcinoma [TCC] or squamous cell carcinoma [SCC]), lymphoma, leukemia, pyelonephritis, infarction, or, rarely, infiltrative RCC.
If imaging suggests renal neoplasm, surgery is usually recommended, although the role of percutaneous renal mass biopsy is evolving. Specifically, biopsy may be indicated when secondary renal tumor is a consideration, when RCC subtype influences the choice of systemic therapy, or when ablative techniques are used as definitive therapy for RCC.
Treatment
Medical Treatment.
RCC is relatively resistant to radiation therapy and chemotherapy. The key to long-term survival in RCC is prompt tumor detection at an early stage. Advanced RCC carries a poor prognosis, which has changed little in the past few decades.
First-line medical therapy for metastatic RCC consists of the administration of cytokines, specifically interferon-alfa or interleukin-2. These treatments result in a median overall survival of approximately 12 months. There are documented cases of spontaneous regression of RCC in the absence of therapy, but these cases are rare and poorly understood.
Surgical Treatment.
Surgery remains the definitive therapy for early-stage RCC. Classically, the treatment of choice for RCC has been nephrectomy. Nephron-sparing surgery was reserved for patients with bilateral renal tumors, solitary kidney, or renal insufficiency. However, partial nephrectomy is being performed with increasing frequency to preserve renal function. In patients with small, solitary tumors the local recurrence rate is 2% or less, similar to the percentage of patients developing contralateral RCC after radical nephrectomy. In patients with contraindications to surgery, local ablative techniques are indicated.
- •
A chest radiograph is usually sufficient in the initial staging of RCC if the primary tumor is small. If the chest radiograph is abnormal or the primary tumor is large, a chest CT should be performed to exclude metastases.
- •
Renal biopsy may be performed safely and has an evolving role in the evaluation of indeterminate renal masses.
- •
Image-guided tumor ablation has become an important alternative treatment for RCC in poor surgical candidates.
Urothelial Carcinomas
Urothelial neoplasms arise from the urothelial lining of the collecting system. Ninety percent of urothelial neoplasms are TCC. Most of the remaining 10% are SCC. TCC accounts for up to 10% of the neoplasms of the upper urinary tract. Of these, approximately 25% directly invade renal parenchyma.
Over 90% of TCCs develop in the urinary bladder and only 5% of urothelial tumors develop in the upper tract. Most upper tract cases occur in the renal pelvis and, less commonly, the infundibulocalyceal portion of the collecting system. In patients with TCC of the renal pelvis, 30% have multicentric disease.
Similarly, SCC of the urothelium is much more common in the bladder than in the upper tract.
Etiology
Risk factors for TCC include advanced age, male gender, and smoking. Multiple chemical carcinogens are also associated with TCC, including aniline, benzidine, aromatic amine, azo dyes, cyclophosphamide and its metabolites, and heavy caffeine intake. Analgesic abuse predisposes to TCC, as does urinary stasis. Other risk factors include Balkan nephropathy, ureteral pseudodiverticulosis, and the hereditary nonpolyposis colon cancer syndromes. SCC of the urothelium occurs in patients with chronic urothelial irritation, which may be secondary to chronic indwelling Foley catheter, chronic urinary calculi, or schistosomiasis.
Prevalence and Epidemiology
TCC is rarely seen in persons younger than age 50, usually occurring in the sixth and seventh decades of life, with a male-to-female ratio of 3 : 1. Bilateral lesions are seen in 2% to 4% of cases.
Clinical Presentation
Gross or microscopic hematuria (75%) is commonly seen in patients with upper tract TCC, and flank pain or acute renal colic is the presenting symptom in approximately 30%. Weight loss and urinary frequency are less common. Of the cases, 10% to 15% are asymptomatic.
Of patients with upper tract TCC, 50% have synchronous or metachronous bladder tumors. Conversely, patients with bladder TCC show upper tract TCC only 0.7% to 4% of the time. In these patients, higher-grade, incompletely excised, or multifocal tumors increase the risk for development of upper tract lesions. Upper tract lesions that develop after cystectomy tend to be more aggressive with worse prognosis; thus, routine postoperative surveillance is performed.
Pathology
TCC manifests in two morphologic patterns: superficial papillary lesions and invasive tumors. In the kidney, TCC appears as a soft tissue mass centered in the renal sinus. It may invade the renal parenchyma, obliterating the renal sinus fat. Renal TCC and SCC are indistinguishable on imaging.
Imaging
Radiography.
Excretory urography has classically been used for the detection and characterization of upper urinary tract anomalies, although sensitivity is limited, ranging from 43% to 64%. TCC appears as a single or multiple filling defects. A classic sign of upper tract TCC is a markedly distended, tumor-filled calyx referred to as an oncocalyx. The surface of the filling defect may be irregular, stippled, or frondlike, depending on the shape of the tumor. Alternatively, TCC may cause obstruction or stenosis of a calyx or infundibulum, resulting in the “phantom calyx” or “amputated calyx,” mimicking renal tuberculosis. The characteristic findings of TCC on excretory urography may also be demonstrated with retrograde pyelography ( Figure 63-18 ). In addition, the goblet sign or champagne glass sign of ureteral TCC, first described on retrograde pyelography, may be demonstrated—this is a cup-shaped dilated segment of ureter distal to a polypoid intraluminal filling defect.
Computed Tomography.
CT urography is the imaging modality of choice for the detection and staging of renal TCC ( Table 63-5 ).
| Modality | Accuracy | Limitations | Pitfalls |
|---|---|---|---|
| Radiography | Poor | Insensitive, nonspecific Requires ionizing radiation | Only detects large renal masses that distort the renal contour Misses anterior, posterior, infiltrative lesions |
| Excretory urography | Poor | Insensitive, nonspecific Requires ionizing radiation and intravenous contrast | Only detects large renal masses that distort the renal contour |
| Angiography | Poor | Invasive Nonspecific Requires ionizing radiation and intravenous contrast | Only detects large renal masses that distort the renal contour Transitional and squamous cell carcinoma not typically hypervascular |
| CT | 91%-94% sensitive | Requires ionizing radiation and intravenous contrast Small mass characterization difficult | Requires good opacification of the collecting system and therefore adequate excretory function |
| MRI | 74%-88% sensitive | Expensive Lack of availability Ideally, requires intravenous gadolinium | Calcifications not well visualized |
| Ultrasonography | Specific data are not available to specify accuracy | Limited detection in obese Operator dependent | Small lesions may be undetected May only detect tumor that obstructs collecting system |
| Nuclear medicine | Specific data are not available to specify accuracy | Poor spatial resolution Requires ionizing radiation | |
| PET/CT | Specific data are not available to specify accuracy | Requires ionizing radiation Expensive Lack of availability | Urinary excretion of fluorodeoxyglucose may obscure a primary urothelial tumor |
Renal TCC and SCC appear as central soft tissue masses located in the renal sinus. Although the mass may be difficult to appreciate, it can obscure the normal renal hilar and pyramidal anatomy, displacing normal renal sinus fat to produce the so-called “faceless kidney” ( Figure 63-19 ). TCC in a calyx or renal pelvis appears as a sessile or polypoid filling defect or as diffuse thickening of the urothelial lining ( Figure 63-20 ).
Renal TCC is isodense to slightly hyperdense relative to renal parenchyma on unenhanced CT. It enhances less than normal renal parenchyma. Calcification is uncommon, occurring in less than 2% of cases.
CT is superior to excretory urography in staging renal TCC because extramural extension and regional nodal metastases may be seen.
Magnetic Resonance Imaging.
Renal TCC shows decreased enhancement compared with renal parenchyma. The lesions are isointense to slightly hypointense on T1-weighted images and isointense to slightly hyperintense on T2-weighted images. Heavily T2-weighted images often show hydronephrosis secondary to obstruction and may demonstrate the tumor as a filling defect ( Figure 63-21 ). The accuracy of MRI in detection of TCC (74% to 88%) is slightly less than CT urography (89% to 100%), and MR urography should be reserved for cases in which CT is contraindicated.
Ultrasonography.
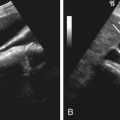
Ultrasonography is generally insensitive to renal TCC. Infiltrating central tumors show obliteration of the normal echogenic renal sinus fat. Diffuse hydronephrosis or focal caliectasis may be seen secondary to urinary obstruction ( Figure 63-22 ). Generally, TCC is hyperechoic relative to renal parenchyma.
Positron Emission Tomography With Computed Tomography.
FDG-PET is not well suited to the evaluation of urothelial malignancy owing to the urinary excretion of FDG, which obscures FDG uptake by the primary tumor.
Differential Diagnosis
The differential diagnosis of an intraluminal filling defect in the renal pelvis includes urothelial tumor, calculus, blood clot, sloughed papilla, fungus ball, air bubble, and debris. The differential diagnosis of an infiltrative renal mass with preservation of the normal reniform shape of the kidney includes TCC, SCC, renal lymphoma, infiltrative RCC, metastases, and inflammatory conditions, such as pyelonephritis, renal tuberculosis, and retroperitoneal fibrosis. An infiltrative renal mass is most likely to represent TCC.
TCC is typically hypodense relative to other collecting system filling defects, including clot (40 to 80 HU) or calculus (>100 HU).
Attenuation values of a collecting system filling defect may differentiate neoplasm from other causes. Urine bacteriologic studies may help differentiate urinary tuberculosis from TCC. Urine cytology is often used to help diagnose TCC but shows only 25% to 59% sensitivity. Additional noninvasive laboratory urinalyses for tumor markers may be combined with urine cytology to increase detection rates.
In any case with suspicion for TCC, tissue diagnosis is necessary. Biopsy may be performed percutaneously with image guidance or ureteroscopically.
Treatment
Medical Treatment.
The role of adjuvant topical chemotherapeutic or immunotherapeutic agents in upper tract TCC after conservative tumor excision is unclear but gaining popularity given positive results with bladder TCC. Current treatment regimens include use of bacille Calmette-Guérin, mitomycin-C, thiotepa, or doxorubicin instilled via the bladder with a ureteral stent in place.
Chemotherapy in advanced TCC uses the MVAC regimen (methotrexate, vincristine, doxorubicin [Adriamycin], and cisplatin). The overall response rate is reported to be 54% with durable response rates as low as 5% to 10%.
Surgical Treatment.
Standard surgical treatment for a unilateral upper tract TCC is nephroureterectomy, including resection of the bladder cuff surrounding the ipsilateral ureterovesical junction. The rationale for this treatment is the high frequency of multifocal tumor and ipsilateral recurrence and the low incidence of contralateral disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree