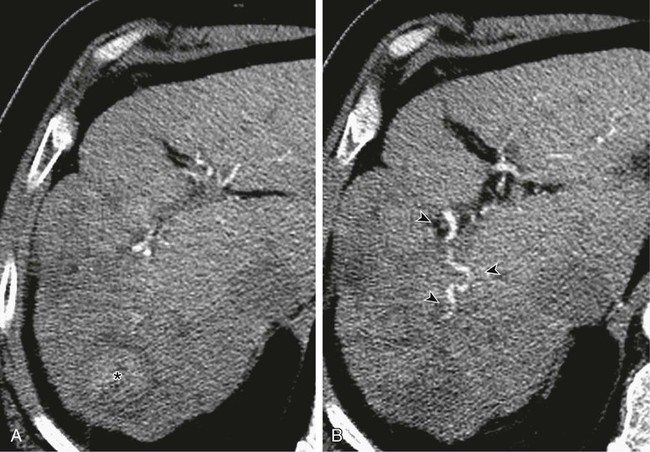
Although surgery remains the best hope for cure in patients with most primary and metastatic liver cancers, many patients are not candidates for resection at presentation. This may be due to extent or distribution of disease, underlying liver function, or general medical condition of the patient. The basic physiologic principle that makes hepatic intraarterial therapy feasible is the dual blood supply to the liver. The portal vein provides more than 75% of blood flow to the normal hepatic parenchyma and is the primary trophic blood supply. Conversely, studies performed in the early 1950s established that the primary blood supply to liver tumors was from the hepatic artery.1 Malignant tumors may be targeted by delivering treatment intraarterially, sparing normal hepatic parenchyma and diminishing or eliminating systemic effects of treatment. In the last 30 years, many papers have been published describing myriad different techniques for performing hepatic embolotherapy. Many of the studies used chemotherapeutic agents and Lipiodol, despite the lack of convincing pharmacokinetic data. Studies clearly demonstrating an increased concentration of the chemotherapeutic agent(s) used for embolization within the embolized tumor(s) were performed with mitomycin C, doxorubicin, and aclarubicin dissolved in hydrocarbon solvents and then in Lipiodol2 or with a lipophilic agent,3 methods that are not used clinically. When the chemotherapeutic agent was dissolved in water and then mixed with Lipiodol and administered as an emulsion, the concentration of drug in the tumor was high immediately but low at 6 hours, 1 day, and 7 days.2 In a study by Raoul and colleagues,4 doxorubicin was given to patients intraarterially, either alone as an infusion, emulsified with Lipiodol, or with Lipiodol and gelatin sponge. There was no significant difference in total amount of doxorubicin released into circulating blood, but patients in whom gelatin sponge was used had less released within the first hour of treatment. In a prospective randomized clinical study evaluating the effect of Lipiodol added to intraarterial cisplatin and doxorubicin, no difference in response was noted between the groups given only intraarterial chemotherapy and those who received chemotherapy emulsified with Lipiodol.5 Another study of intraarterial doxorubicin versus doxorubicin with Lipiodol found no difference in the area under the concentration time curve (AUC) or the terminal half-life, and no difference in pharmacokinetic or systemic toxicity in the same dose schedule as in patients who received intravenous doxorubicin.6 It seems that the pharmacokinetic data supporting the use of intraarterial chemotherapy or chemotherapy plus Lipiodol, at least when given with the hydrophilic chemotherapeutic agents commonly used today and administered as an emulsion, are not robust. What has been demonstrated in both pharmacokinetic and clinical studies4,7,8 is the benefit derived from addition of an embolic agent, even Gelfoam, to the transarterial chemoembolization (TACE) cocktail. Some authors even postulate that the primary effect of embolotherapy is derived from the embolic agent and not the chemotherapy or Lipiodol.8 If indeed the primary effect of TACE results from ischemia-induced cell death, the goal must be to induce terminal vessel blockade, inasmuch as we know that more proximal vessel occlusion within the liver leads to the almost immediate development of flow distal to the occlusion via myriad collateral vessels, as demonstrated by Michels in 1953.9 Bland embolization with small particles is an effective method of intraarterial treatment of hypervascular primary and metastatic tumors to the liver that can be used instead of TACE, thereby avoiding the added expense and systemic toxicity of chemotherapy. Bland embolization is indicated for treatment of unresectable “hypervascular” hepatic neoplasms and can be used to treat both benign10 and malignant11 processes, but malignant lesions are most commonly treated. It is important to define “hypervascular” hepatic tumors. Malignant tumors in the liver derive primary blood supply from the hepatic artery, whereas normal hepatic parenchyma is primarily supplied by the portal vein. The term hypervascular does not simply mean the tumor has arterial blood supply, but rather that the tumor is more “arterialized” than normal liver. This encompasses tumors that demonstrate enhancement on the arterial phase of a triple-phase computed tomography (CT) scan even if these same tumors appear to be low density on the equilibrium phase. Although there are no absolute contraindications to hepatic arterial embolization, in general, patients with Child class C cirrhosis should not undergo embolization as a primary method of treatment; their survival is much more likely to be determined by their liver disease, not their tumor. If embolization is warranted, such as to stop bleeding, embolization should be limited in scope. When evaluating a patient for embolization, one should consider the severity of the underlying liver disease as well as the extent of the tumor being treated. Several methods of classification have been developed in an effort to weigh both these factors, the simplest being the Okuda stage. In this scheme, patients are given points for the presence or absence of ascites, serum bilirubin and albumin levels, and tumor extent. In 1999, Llovett et al.12 proposed the BCLC (Barcelona Clinic Liver Cancer) staging classification as a means of both classifying patients and linking their stage to a specific treatment. Although selective embolization of a solitary well-circumscribed HCC in a patient with Child class B cirrhosis might be well tolerated, embolization of a hemiliver in a Child A patient with multifocal hepatoma involving more than 75% of the liver and with portal vein tumor thrombus may result in hepatic failure and even death. Bland hepatic arterial embolization does not require anything not traditionally found in the usual angiography suite. Selective catheters, such as 4F or 5F Cobras and reverse-curve catheters (Simmons or SOS catheters), are used through a vascular sheath placed in the right external iliac artery. Coaxial microcatheters are used when necessary for subselective embolization. Conventional nonionic contrast material is typically used. Since treatment depends on terminal tumor vessel blockade, we use the smallest particles commercially available. Until the late 1990s, 50-µm polyvinyl alcohol (PVA) particles (Cook Medical, Bloomington, Ind.) were the smallest particles available, then Embospheres (BioSphere Medical, Rockland, Mass.), a hydrophilic trisacryl gelatin microsphere, became available in 100- to 300-µm sizes. Despite the larger sphere size, the fact that the microspheres were spherical and hydrophilic meant they did not “clump” like PVA and were capable of penetrating more distally into the terminal vasculature.13–15 Eventually, 40- to 120-µm Embospheres became available, and we began to use graduated sizes of Embospheres almost exclusively. Use of Embospheres is associated with preservation of arterial patency,16 an important consideration in patients who are expected to need repeated treatment. As part of the preprocedure workup, every patient has a triple-phase CT scan within 1 month of the scheduled embolization. Triple-phase CT is essential for documenting the extent of disease, demonstrating arterial anatomy, evaluating the portal venous system, and looking for nonhepatic blood supply to the tumor. This study serves as the basis for a treatment plan. The extent and distribution of the tumor are laid out (Fig. 68-1, A), arterial blood supply to the tumor is evident (Fig. 68-1, B), and any contribution from the extrahepatic vasculature, such as the phrenic (Fig. 68-2, A and B) or internal mammary arteries, should be seen. On the day of the procedure, the plan needs only to be executed.
Bland Embolization for Hepatic Malignancies
Indications
Contraindications
Equipment
Technique
Preprocedure Preparation
Anatomy and Approach
Bland Embolization for Hepatic Malignancies