4 | Blood Flow Analysis During Pregnancy |
This section will describe the physiological and pathological factors influencing blood flow in individual vascular areas during pregnancy.
Uteroplacental Vessels
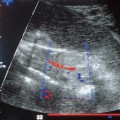
The waveforms of the uteroplacental aa. should be examined qualitatively, since quantitative determination of the blood supply to the uterus and the intervillous space is difficult (Fig. 4.1). Because other maternal vessels are close by, vessels must be localized selectively. Not only can the Doppler window be placed in the wrong vessel, but the Doppler sonograms of different vessels may be superimposed on each other. This is not always easy to spot (Fig.4.2). Two criteria must be evaluated:
1. Indices of the systolic/diastolic velocity changes, which are a measure of the impedance in the downstream vascular beds, and hence an indication of impaired blood flow into the intervillous space.
2. A postsystolic notch in the waveform, which is an indication of an incomplete trophoblast invasion (Fig. 4.3). The notch shows the persistence of pulse wave reflections when the spiral aa. do not dilate, i. e., are incompletely fetalized.
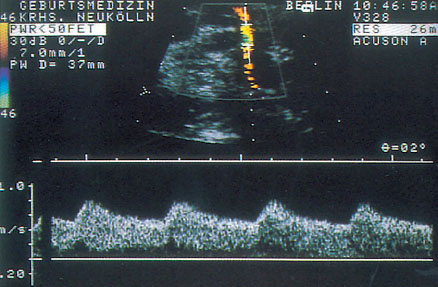
Fig. 4.1 Doppler sonogram of the uterine a.
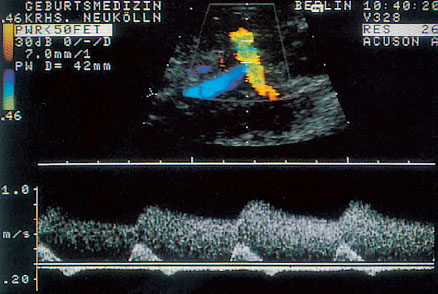
Fig. 4.2 Doppler sonogram of uterine a. superimposed on the iliac a.
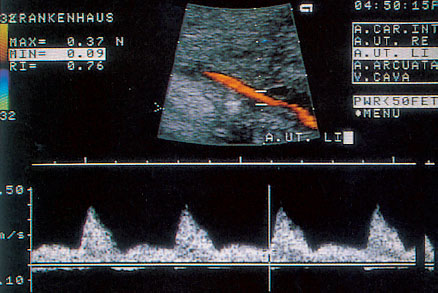
Fig. 4.3 Doppler sonogram of uterine a. with postsystolic notch.
Reference Values
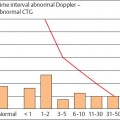
During an uncomplicated pregnancy the end-diastolic flow in the uterine aa. or the arcuate aa. is >50% of systolic peak maximal velocity (Fig. 4.4). The resistance index (RI) is nearly constant at 0.35 after the 20th week of pregnancy, with a maximal value near RI = 0.50. After the middle of pregnancy a persistent postsystolic notch, especially on the placental side of the uterus must be considered to be abnormal.
Physiological Flow Changes
Brief maternal exertion may be followed by an increase in impedance in the uteroplacental aa., which may be enhanced in a complicated pregnancy (Campbell and Cohen-Overbeek 1987).
Uterine contractions are accompanied by reduction of the blood supply to the intervillous space (Fig. 4.5) (Fendel and Sohn 1989).
Studies of the uteroplacental aa. showed marked reduction in flow velocities, especially in diastole, without a dicrotic late systolic notch in a previously normal waveform (Fendel 1986, Fendel et al. 1984, 1986, 1987, Fleischer et al. 1987, Janbu et al. 1985). This is probably due to summation. Experimental data suggest that during a contraction some of the vessels piercing the myometrium are completely compressed (Borrell et al. 1965). Medications that delay labor may contribute to increased diastolic blood flow.
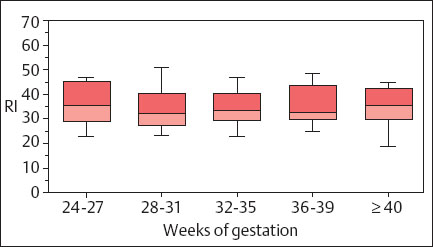
Fig. 4.4 Changes in the RI of a uteroplacental a. during pregnancy. Box and whisker plot. Boxes: 25th, 50th, and 75th percentiles; whiskers 10th and 90th percentile (reproduced from Vetter 1991b).
The position of the mother during the procedure influences the pulsatility index (PI) of the uterine a. The PI falls significantly when changing from the supine to the left lateral position. The explanation for this may be that the contractions diminish at the same time, suggesting an indirect effect of the tone of the uterine wall, which is dependent on position (Park and Hidaka 1991).
Brief maternal heat stress, for example, in a sauna, does not lead to flow changes in the uteroplacental and fetoplacental aa. Only in a few cases where the blood pressure fell could a rise in the S/D ratio be demonstrated (Vaha et al. 1991).
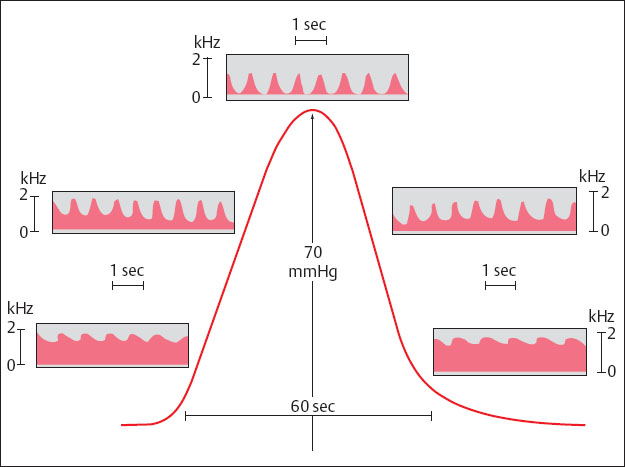
Fig. 4.5 Doppler sonogram of a utero-placental a. during a contraction (reproduced from Fendel and Sohn 1989).
Abnormal Flow Changes
Vascularization of the placental bed is marked not only by trophoblast invasion (Pijnenborg et al. 1980), but also by considerable dilatation of the vascular lumina due to humoral factors. High levels of estrogens have been identified as one of the responsible factors (Moll et al. 1988). Their mode of action is probably a rise in nitrogen monoxide (NO) (Campbell 1993). This would explain the rise in the diastolic flow velocities in the uteroplacental aa. that may be seen from the beginning of the second trimester, and the disappearance of the late systolic notch in the waveform by the 26th week of pregnancy at the latest (Fleischer et al. 1986). If these necessary anatomical adaptations to an increased demand for perfusion do not occur, the pregnancy is at risk. Often the result is preeclampsia, or pregnancy-induced hypertension (PIH) (Brosens 1977), or intrauterine growth retardation (IUGR) resulting from the placental hypoperfusion (Campbell et al. 1983, Cohen-Overbeek et al. 1985, Hackett et al. 1986). Occlusive vascular lesions in the spiral aa. can lead to a similar result (Sheppard and Bonnar 1980). A close connection between changes in the placental bed and those in the uteroplacental flow curves has been demonstrated by postpartum biopsies of the placental bed (Voigt and Becker 1992). Vascular spasms or morphological changes in the vessels are marked by a clearly elevated flow impedance in the vessels (Fleischer et al. 1986, Trudinger et al. 1985, Vetter et al 1986).
Medications
β1-sympathomimetic drugs probably facilitate diastolic blood flow by reducing uterine muscle tone (Vetter et al. 1989). On the other hand, atenolol, a selective β1-blocker, raises the PI in the uteroplacental vascular bed and in the fetal aorta if blood flow volume in the aorta and the umbilical v. is maintained (Montan et al. 1987).
Fetoplacental Vessels
Umbilical Vessels
Blood flow in the umbilical aa. is analyzed qualitatively, because quantitative analysis is impractical due to problems with the presence of two vessels (Fig. 4.6). The evaluation covers systolic/diastolic changes. Elevated flows are taken as a sign of adequate villous stem vessel architecture. As pregnancy progresses, the villi mature and impedance declines, leading to an increase in diastolic flow rate (Trudinger 1987). The major decline in pressure responsible for this occurs in the small arteries and arterioles of the tertiary villi (Becker 1981).
Reference Values
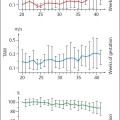
The RI of the umbilical aa. tends to diminish as pregnancy progresses, declining from ca. 0.70, maximally 0.80 at 24 weeks of gestation, to 0.55, maximally 0.65 at term. The values then remain constant (Fig.4.7).
Early in pregnancy diastolic flow is absent in all cases up to the 10th week of pregnancy. The proportion of fetuses in whom diastolic flow is present then rises continuously to 100% at week 15 (Arduini and Rizzo 1991, Jauniaux et al. 1992).
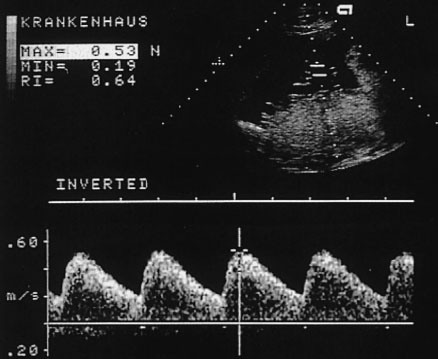
Fig. 4.6 Doppler sonogram of an umbilical a.
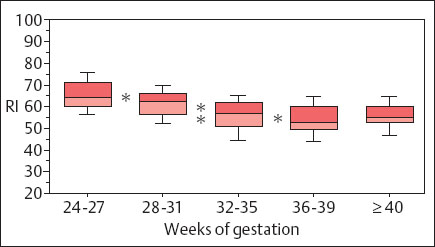
Fig. 4.7 Changes in the resistance index of an umbilical a. during pregnancy. Box and whisker plot. Boxes: 25th, 50th, and 75th percentiles; whiskers 10th and 90th percentile. Confidence level: * p < 0.05, * * p < 0.01 (reproduced from Vetter 1991b).
Physiological Flow Changes
The waveforms of the umbilical aa. are quite variable. For one thing each artery may supply a terminal bed that differs materially from the other. Therefore, before prematurely interpreting marked fluctuations between two readings, the two arteries should be analyzed separately. But even fetal factors can influence the extent of these fluctuations materially. Movements of the extremities or respiratory movements are accompanied by noticeable changes in the flow curves (van der Mooren et al. 1991).
Some indications of increased impedance in the fetoplacental circulation have been found when the mother was supine (Marx et al. 1986). This led to the hypothesis of a “sluice flow” in the villous stem vessels. The flow was thought to be brought about by compression of vessels resulting from backflow from the dilated intervillous space. Other authors were unable to confirm any change in umbilical blood flow (Fitzgerald et al. 1984, Park and Hidaka 1991). There is no change in the fetoplacental circulation in a healthy pregnant woman when standing. A rise in the S/D ratio was only found in hypertensive gravid women, especially those in whom an increase in peripheral resistance had been ascertained (Sørensen et al. 1992).
Elevated maternal blood pressure readings without changes in the placenta or fetus are accompanied by normal flow patterns in the umbilical aa. (Fitzgerald et al. 1984, Fleischer et al. 1986, Milliez et al. 1983).
Changes in the total viscosity of cord blood have at most a minor influence on umbilical a. impedance (Steel et al. 1991). Even when plasma viscosity was examined separately no connection with the Doppler sonogram could be found (Fairlie et al. 1991).
Small changes in oxygen supply do not influence the flow indices in the fetal and fetoplacental vessels significantly (Meyenburg et al. 1991).
Pathological Flow Changes
Deviations from the norm in the flow curve are an indication of changes in the placental vessels. The total developmental disorders and pathological changes in the vessels must be considerable to be detectable in the waveform. In animal experiments at least half the placental vessels had to be embolized before the PI rose (Muijsers et al. 1991). One should also note that areas of infarction can have no further influence on the waveform. They are invisible to Doppler sonography.
Pressure changes, however, do seem to have an influence on blood flow through the umbilical aa.: A reduction in the amount of amniotic fluid of itself can increase the impedance in the fetoplacental circulation, probably by mechanical compression. In pronounced oligohydramnion the rise in impedance in the umbilical aa. was suppressed temporarily by instilling fluid (Wladimiroff 1988).
Morphological Changes
Morphological changes in the fetoplacental bed correlate with changes in the course of flow velocity changes in the umbilical aa. (Giles et al. 1985, Jimenez et al. 1988, McCowan et al. 1987). Such changes may come about primarily in the fetal vessels, but they may also be a consequence of uteroplacental problems. In such cases diastolic flow velocities decline. Histologically, normal Doppler sonograms correspond either to normal vascular and villous architecture, or to focal lesions compensated by degenerative vascular disease or growth. In the absence of compensatory changes, diastolic flow in the umbilical aa. diminishes (Hitschold et al. 1992, Nordenvall et al. 1991). In fetuses with growth retardation and normal Doppler sonogram vascularization in the terminal villi was found to take its normal course (Hitschold et al. 1993).
The unusual biological situation of multiple pregnancy in a woman with several gestational sacs induced Giles et al. (1993) to look for disturbances in blood supply. The distinguishing criteria were blood flow and S/D ratio on the one hand, and proportion of small arteries in the placenta on the other. There were clear-cut differences in microvascular supply between siblings with different Doppler sonograms. The authors interpreted this finding as suggesting that vascular problems derive from the fetal rather than the uteroplacental circulation. This interpretation needs confirmation.
Extreme changes in the waveform present a separate category: diastolic or end-diastolic flow may be absent (Fig. 4.8) or even reversed (Fig. 4.9) (absent or reversed diastolic flow = ARED flow). These are signs of significant or even extreme general change in the placental circulation. Reverse flow signifies that a part of the blood between the fetus and the placenta simply oscillates between fetus and placenta. In sum such flow patterns are “ominous signs of grave impairment of fetal blood supply” (Battaglia et al. 1993) or warnings of “catastrophic perinatal end-results” (Brar and Platt 1988).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree