MR imaging has had a major impact on understanding the dynamic neuropathologic findings of multiple sclerosis (MS), early diagnosis of the disease, and clinical trial conduct. The next 10 years can be expected to see further advances with a greater emphasis on large multicenter studies, new techniques and hardware allowing greater imaging sensitivity and resolution, and the exploitation of positron emission tomography molecular imaging for MS. The impact should be felt with a new emphasis on gray matter disease and processes of repair. With new ways of monitoring the disease, new treatment targets should become practical, helping to translate advances in the understanding of immunology and regenerative medicine into novel therapies.
Over the past 3 decades, MR imaging has become a primary tool for the investigation of multiple sclerosis (MS) and for clinical diagnosis. A key milestone was passed with introduction of the McDonald criteria, which established specific criteria for MR imaging–supported diagnosis. More recent criteria have expanded the applications, scope, and potential sensitivity to allow a reliable diagnosis to be made within a year of initial presentation, even without use of contrast agents. This incorporation of MR imaging into routine clinical practice has been a major driver for past development of MS imaging.
Another driver has been the role of MR imaging as a disease activity and progression biomarker in new drug development. MR imaging changes correlate well with neuropathologic features. Many studies have confirmed correlations between acute gadolinium (Gd) enhancement of T1-weighted MR imaging and relapse or chronic structural changes (eg, brain atrophy) and clinical progression. This has led to acceptance of MR imaging as a near surrogate marker in recent clinical trials of anti-inflammatory therapies for MS.
The development of new types of MR imaging contrast, advances in radiofrequency coil design, and the availability of higher magnet field strengths provide a greater range of imaging measures with higher sensitivity for neuropathologic changes and greater anatomic resolution. The development of a wide range of software tools now widely disseminated publicly (eg, ) and commercially enables imagers with even limited specialist analytic resources to apply increasingly sophisticated approaches for quantitative data interpretation. Coupled to advances in information technology (IT), a new quantitative neuroradiology of MS has emerged. This already has had an impact on clinical trials. It holds additional promise as an approach to improved precision of individual prognosis or assessments of clinical therapeutic responses.
Ten years is a surprisingly short time. This author is also aware of the difficulties of accurately looking to the future even this near, however. Nonetheless, current trends in imaging science suggest some specific types of applications development that appear likely. Potential advances in the application of MR imaging techniques are considered. The great opportunities for molecular imaging, particularly for increasing understanding of the dynamics of MS pathologic findings and in support of early development of neurotherapeutics, are outlined.
Advances in MR imaging
Toward the Goal of “Personalized Medicine”
One of the most striking features of MS is the heterogeneity of expression. Heterogeneity of neuropathologic findings mirrors the heterogeneity in the clinical course. Lucchinetti and colleagues have proposed four distinct pathologic forms of the disease. These differences suggest rational hypotheses concerning the major differences in prognosis and responses to therapy that are likely to be tested in coming years. With increasing use of advanced imaging tools that allow coregistration of images acquired with different contrasts, explicitly multivariate analyses of regional pathologic changes are possible. Classifiers based on these multivariate features can be used in vivo in ways that are similar to the ways in which conventional histologic stains are used ex vivo.
Stratification of patients on the basis of imaging neuropathologic findings (in combination with other approaches) should be explored for better targeting of treatments. Differentiation of drugs in the market is a key goal for pharmaceutical companies. Clear demonstrations of new drug value are being demanded by payers. In this environment, there is an opportunity to couple the use of imaging methods that speed proof of pharmacology in early drug development to stratification of responder populations. MS is an ideal “test case” for this new way of working, given the range of aspects of the disease process that can be imaged even now.
A related aspect of personalized medicine is to define when treatments have achieved their goals or are no longer having a major impact on disease processes in individual patients. The clinical significance of neutralizing antibodies with interferon treatments, for example, can be resolved with more data relating the nature and magnitude of antibody responses and MR imaging–assessed new disease activity using standardized approaches. Although serial MR imaging studies have not been an effective way of monitoring treatment in the past because of the relatively modest effects of the drugs, as more effective treatments become available, quantitative criteria for determining treatment evaluation periods even for individual patients should be able to be formulated.
Harnessing the Power of Large Studies
A major enabler for an evidence-based quantitative approach to clinical decision making based on imaging neuropathologic findings is likely to be more large well-powered outcome studies. Because resources for new investigations are always limited, however, the opportunity to build these on a stronger foundation of imaging IT integration within health centers needs to be seized. Needs of imaging for clinical care and imaging for research evaluation of patients can be met together. There is an increasing potential for standardization among even sophisticated imaging protocols. Observational studies can be constructed based on data derived from other research activities or usual clinical practice. Although limited to submitted clinical trials data sets and centralized (rather than having archiving distributed at different centers providing data), the Sylvia Laurie Center provides a model of how this kind of activity can generate substantial new value, although the statistical models used for studies must appropriately take into account the biases that can be introduced by this kind of retrospective databasing.
The potential for this kind of “e-science” should be enhanced as electronic patient records are used more widely. The technical problems associated with large-scale electronic archiving of image data were solved over the past decade, and there already is widespread use of conventional picture archiving and communication (PACS) systems in filmless neuroradiology units. Improvements to the software environment and hardware capacities may be expected to enable full integration of these data with other patient records information in common databasing frameworks. This should allow clinical outcomes and imaging data to be related rapidly within any single institution. With care to ensure that all personally identifiable information is removed, these data can be shared among researchers in different institutions to facilitate the kinds of large meta-analyses needed to understand trends in a heterogeneous disease.
More ambitious population-based imaging studies should become more common as this value for other types of disease epidemiology is realized. A recent study of extended families in Sardinia illustrates the value of this type of study, with the demonstration of significant expression of white matter abnormalities in asymptomatic first-degree relatives of patients who had MS. Biobank efforts underway in several countries to chart the risk factors and extended prodromes for common diseases by long-term monitoring of extremely large (500,000+ persons) healthy populations should provide an entirely new kind of information. Although thus far, these efforts have been directed toward follow-up of a middle-aged population, they may be expected to be expanded into earlier stages of the life cycle and into periods when the risks for developing MS are higher.
Software for high-quality unsupervised image analysis must be a major development focus to enable this type of work. Already, academic laboratories have developed tools that are relatively robust to minor variations in acquisition techniques, allowing increasingly automated approaches to the derivation of specific metrics from imaging data sets. With some improvements, these should allow large archived primary data sources to be mined rapidly for specific metrics. Collaborative multicenter archiving should facilitate the discovery and qualification of measures with sufficient power to encourage their rapid acceptance by the imaging community.
Imaging Genomics
By increasing potential study size, all these developments allow a qualitatively new type of experiment. Plausibly sufficient power has become available for testing genetic hypotheses utilizing brain structural (and, potentially, functional) changes using imaging as an intermediate or “endo”-phenotype, for which a greater proportion of population variance can be explained by single gene differences than with usual clinical features.
One recent study has provided evidence for an association between the brain-derived neurotrophic factor met66 allele and lower degrees of neocortical gray matter atrophy and T2 lesion volume in patients. Another study has suggested a relation between functional polymorphisms in the CCR5 and CCO5 ligand/receptor pair and worse clinical disease course and altered T2 and T1 lesion loads on MR imaging. The CCR5 303∗G polymorphism was associated with reduced T2 hyperintense and T1 hypointense lesion volumes.
Critical review of these findings suggests that although of great interest, they need to be replicated before they can be accepted as more than hypotheses. The numbers of plausible gene associations are huge across different disease subphenotypes. This brings considerable potential for false-positive results. What has been lacking thus far in the literature is a full understanding of the number of potential associations that have been tested before the finding of the individually significant changes reported. Much larger populations also are needed for confidence in outcomes, because the contributions of single genes can be expected to be heterogeneous.
Nonetheless, the approach is promising and is potentially a way of directly linking functional and structural imaging to molecular pathologic findings. Obvious extensions of the single polymorphism genetic approach should involve the use of pathway analyses that assess whether clusters of genes with related functions are associated with a feature. Image analysis based on transcriptomic profiling also is to be expected.
New Applications and New MR Imaging Contrast Agents
Preclinical studies have demonstrated the complex dynamics of inflammatory response and the potential for selective imaging of different cell populations and effector molecules. Expression of endothelial inflammatory mediators, cell trafficking, and macrophage infiltration all can be imaged with targeted MR imaging contrast agents. With the development of immune therapies directed against specific aspects of the inflammatory cascade, these measures should ideally be extended to human studies to characterize the response across different clinical syndromes and to provide better pharmacodynamic measures for early-phase drug development.
Ultrasmall iron oxide (FeO) particles have been approved for other indications. Their dextran coating is associated with only a modest rate of (largely minor) adverse events, and more biologically inert alternatives can be expected. Injected particles are engulfed by circulating monocytes before they migrate into a new inflammatory lesion (compelling direct evidence, which has been presented with studies of carotid plaques). The contrast thus provides an index of the dynamics of a specific arm of the antigen-presenting inflammatory cell pool. It provides information complementary to Gd MR imaging, which reports on changes in blood-brain barrier permeability probably reflecting an earlier stage of evolution of a focal lesion.
In principle, lymphocytes could be labeled ex vivo and reinjected, but this kind of experiment is complex and raises safety issues that may be difficult to resolve in a research context. Unfortunately, despite the availability of antibodies directed against surface proteins specific for particular immune cell populations (eg, rituximab) and surface determinants, the potential for widespread use of similar molecules with Gd or FeO labels seems modest. The relatively low sensitivity of conventional MR imaging contrast is one major issue, particularly given that any contrast would need to be administered in low enough doses to preclude significant biologic activity. Amplification of contrast signal or use of ultra-high-field imaging could present a solution, however. A specific concern with antibody-based targeting is that the contrast agent has sufficiently rapid kinetics to clear the blood pool fast enough to allow selective tissue imaging, although this could be managed by appropriate re-engineering. The hurdle of ensuring safety for subjects with such pharmacologically complex agents remains.
Extending the Understanding of Axonal Pathologic Findings
Diffusion MR imaging
A rediscovery of the importance of axonal injury in MS in the 1990s and new recognition of its potentially critical importance in determining disability should undoubtedly lead to further developments in quantitative axonal structural imaging over the next decade.
Diffusion tensor MR imaging is now a well-accepted tool for mapping structural connectivity among brain regions. The fractional anisotropy that characterizes white matter has components that can be related to restricted diffusion of water associated with myelin and with the axoplasm. Diffusion measures are sensitive to demyelination, axonal loss, and probably also to the changes in caliber and axonal internal organization associated with demyelination. Developments in instrumentation and analytic tools are making it much easier to obtain exceptionally high-quality diffusion MR imaging data that can be related directly to anatomic features of white matter and gray matter.
A major issue that has limited development in the field has been accurately comparing myelin organization between individuals and groups. Conventional software tools for brain registration perform white matter registration poorly because of the lack of specific contrast edges to drive anatomically meaningful landmark matching. One advance has been to use tract-based spatial statistics (TBSS). This simple approach uses the fractional anisotropy within an individual brain to develop a white matter “skeleton” reflecting major local trends that can be used as a registration framework. Quantitative variations in the skeleton can be used to contrast spatial changes in white matter across individuals and groups, defining variation in robust statistical terms, and thereby establishing potential diagnostic and prognostic criteria.
An alternative approach has been to characterize changes within anatomically defined white matter. Although this approach is more user-intensive, automation should be possible. Exploration of the distribution of connectivity measures within individual tracts can define potentially sensitive and specific neuropathologic measures. The advantages of diffusion MR imaging lie in the sensitivity to microstructural changes.
Proton magnetic resonance spectroscopy
Axonal pathologic findings also can be defined metabolically. Proton magnetic resonance spectroscopy was the first approach in the modern era to highlight the considerable extent of neuroaxonal injury in the disease. The N-acetyl aspartate (NAA) resonance reflects mitochondrial function. Mitochondria may be impaired early in inflammatory response by immune modulators, such as nitric oxide. There have been suggestions in the literature over the past decade that metabolic changes in the disease are early, precede irreversible loss of axons, and may be reversible. These concepts should be tested and developed further over the next decade.
One report has shown recovery of reduced NAA after introduction of interferon-β treatment. This suggests that neuronal metabolism is improved by reducing inflammation. Reversible neuroaxonal mitochondrial metabolic dysfunction also has been reported in other diseases. Much more needs to be done to develop this exciting notion, however. A first small follow-up study was unable to reproduce the finding with interferon-β, suggesting that there is variation in patient response-related factors, such as disease stage or subtype.
Greater Focus on the Gray Matter
It now is well accepted that gray matter is involved extensively in MS. Demyelinating lesions are common in the cortex and subcortical gray matter. Extended fields of subpial demyelination also can be found across the cortex. These are likely related to nodules of B-cell infiltration in the overlying meninges and may reflect action of diffusing antimyelin antibodies. Atrophy of cortical and subcortical gray matter has been well demonstrated and is progressive through the disease. In the neocortex, this may largely be attributable to a relative neurite dystrophy, but in the subcortical nuclei, such as the thalamus, atrophy is associated with substantial loss of neurons.
Conventional imaging approaches only rarely visualize gray matter inflammatory lesions. This seems, in part, to be a consequence of altered relative reduced contrast between the inflammatory lesions and normal gray matter. Inversion recovery sequences contribute to usable contrast with efforts to improve signal-to-noise with greater image averaging. Limited resolution of conventional images for the thin (2.5 mm or so) neocortex also plays a role, however, and may be expected to be addressed further in the future with use of high-field systems and high-resolution scanning protocols. New imaging sequences are being developed in several laboratories (eg, high-resolution T1 mapping techniques). Further developments are expected.
Because the problem of resolution critically depends on signal-to-noise, the recent availability of 7.0-T imaging for humans may open up new potential for cortical imaging of MS lesions. Recent data suggest that linear in-plane resolution on the order of 250 μm or better should be achievable commonly at this field. Advantages may accrue from the ability to visualize the close relation between small draining veins and inflammatory lesions.
Improved analytic tools should continue to have significant impact for studies of gray matter. Better methods for segmentation of gray matter particularly should help to drive the field forward. If the need for time-consuming manual interventions can be reduced, cortical flattening methods should greatly enable more sensitive quantitative assessment of gray matter changes. The correlations between disability progression and brain atrophy and between brain atrophy and gray matter loss suggest that clinically relevant prognostic measures could be derived. Extensions of the paradigm for cognition also seem possible.
New Range of Clinical-Pathophysiologic Correlations
Perfusion MR imaging
Integrating neuropathologic information into clinically meaningful outcome measures should be enhanced by the use of different types of physiologic summary measures. These can assess tissue function as it is affected by the neuropathologic findings. Brain perfusion provides one such measure. The past few years have seen substantial developments in this area, with availability of robust sequences for noninvasive arterial spin tagging and methods for quantitation of the perfusion changes. Pilot studies have demonstrated that cerebral blood flow is reduced in brains of patients who have MS, consistent with prior positron emission tomography (PET) studies. Perfusion is increased in acute inflammatory foci, however. The arterial spin labeling techniques should be enhanced by the availability of improved flow-sensitive sequences, higher signal-to-noise with phased-array coils, and higher field MR imaging. One goal is to use perfusion MR imaging as a marker “intermediate” between measures of neuropathologic and clinical outcomes. As a measure of function, perfusion MR imaging provides indices of the consequences of pathologic findings, not just locally but in functionally connected regions of brain.
Functional MR imaging
The past several years have also seen growth in applications of functional MR (fMR) imaging to MS. fMR imaging is an indirect measure of aggregate excitation-inhibition in gray matter microcircuits. Perhaps the most important outcome of fMR imaging studies in MS over the past several years has been to highlight brain circuit plasticity and its potentially adaptive role. An argument for the importance of adaptive plasticity as a limit to expression of the neuropathologic findings is particularly compelling for this disease in which irreversible neuroaxonal changes accompany even the early acute inflammatory responses, whereas patients show complete clinical recoveries after relapses. Potentially maladaptive functional changes may be seen, however. Progressive disease pathologic findings also must limit the potential for adaptive capacity.
Although precise interpretation of fMR imaging measures is still needed, fMR imaging applications are likely to grow substantially. Spinal cord fMR imaging has been demonstrated convincingly and promises the potential to assess relative contributions of plasticity at the spinal and cerebral levels to recovery. The approach offers a potential way of objectively assessing neurorehabilitation or other therapies that promote plasticity. Pilot applications suggest the possible utility of extensions of the univariate analytic approaches (regional functional activation measures) to multivariate (eg, functional connectivity) measures that may provide more powerful measures of longer distance integration of brain systems. Encouragingly for applications to the evaluation of therapies, recent work has demonstrated how fMR imaging can be applied with confidence in multicenter protocols.
Myelin repair
Magnetization transfer ratio and short T2 imaging
The past 3 decades have focused attention in MR imaging studies on the primary inflammatory events and their immediate sequelae. A major goal now is to extend understanding of tissue repair and, particularly, remyelination.
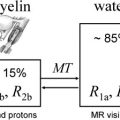
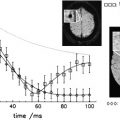
Several studies have now provided compelling demonstrations that multicompartment T2 relaxation provides relatively specific information on myelin content from the short T2 component of the brain water MR imaging signal that is thought to reflect water associated closely with myelin. The extent of local changes in myelin water content with demyelination is large (30%–50%) within macroscopic MR imaging voxels. This suggests that measurement of the myelin water fraction should be viable as a measure of remyelination for assessment of therapies directed at enhancing intrinsic remyelination pathways or at stem cell replacement therapies. Thus far, however, the technique has not been widely used. A major limitation was that only single-slice acquisition was practical. New applications should expand with the availability of multislice or three-dimensional acquisition techniques that allow whole-brain coverage.
Magnetization transfer (MT) MR imaging is an alternative method for assessing characteristics of the water pool associated with myelin. It is sensitive to early changes that can precede the development of Gd-enhancing lesions by many months, and therefore must reflect microscopic pathologic findings of myelin. MT MR imaging (which typically uses whole-brain acquisition) has been related to the evolution of lesions longitudinally in many studies. As expected from the sensitivity to demyelination, MT MR imaging seems sensitive to early remyelination. Chen and colleagues have provided data recently suggesting that the dynamics of remyelination can be monitored quantitatively in individual lesions over the days to weeks after acute relapse. Future research needs to assess whether patterns of recovery in individual patients provide a good prognostic measure and whether the nature of the remyelination response is a useful index of underlying pathologic mechanisms for patient stratification. MT MR imaging also should enable development of remyelination therapies by providing a powerful pharmacodynamic outcome measure for clinical trials.
The two approaches to myelin imaging rely on different contrast mechanisms, and therefore are complementary. Their relative sensitivity and specificity need further exploration. In addition, although substantial qualification has been possible using postmortem material, both rely on making inferences about relations between molecular dynamics reflected in biophysical phenomena and pathologic findings that are indirect. Availability of a specifically targeted molecular imaging marker of myelin would be ideal.
Molecular imaging
Another route to an improved understanding of the dynamic molecular pathologic findings of MS is to make use of exquisitely sensitive PET methods to characterize molecular changes directly. PET has been applied for some years for metabolic studies in MS and in other many neurologic diseases. The cerebral metabolic rate for oxidation of the preferred brain substrate, glucose (CMR glu ), can be measured with injection of positron-emitting 18 F-2-fluro-2-deoxy-D-glucose (FDG). Brain oxidative metabolism is coupled closely to the closely coupled neuronal and glial metabolic changes associated with neurotransmitter release. In patients who have MS, reductions in the global metabolic rate for glucose are most pronounced in the prefrontal cortex and in specific subcortical regions, including the hippocampus and thalamus. Progressive reductions are seen over time, suggesting a close relation between metabolism and the changes in neuronal activity contributing to longer term disability.
Focal inflammatory lesions show increased glucose metabolism as a consequence of the increased glycolytic rates of inflammatory cells. These changes can be defined in white matter and are associated with acute relapses. Molecularly specific measures of the focal immune response also are possible because of the high sensitivity of PET. PK11195 is an isoquinoline that binds relatively specifically to the peripheral benzodiazepine receptor (PBR; translocator protein 18KDa). PBR is an outer membrane protein of mitochondria that is located predominantly in glial cells (astrocytes and microglia). Increased PK11195 binding reflecting increased PBR protein expression is found in MS. This up-regulation may reflect an increase in mitochondrial number or an increase in the number of PBR sites of mitochondria with activation of cells. In practice, it may be able to be interpreted as a marker for activated microglia in the brain.
A pilot study demonstrated uptake of PK11195 within the sites of active MS lesions defined by MR imaging. More comprehensive follow-up with in vitro pathologic correlations indicated that although increases in PBR expression were found in regions of focal inflammatory pathologic change, changes also were seen in the cerebral gray matter and projection areas of lesioned white matter, which is potentially consistent with a microglial reaction after axonal transection.
Additional tracer molecules have been described that also bind to the PBR receptor (eg, PBR28, vinpocetine) but that have potentially more favorable imaging characteristics. Robust interpretation of results demands validation of modeling methods for specific signal detection. These are now being better defined. Over the next 10 years, the applications of these molecules for understanding the nature of the innate immune response in MS should thus increase. This should open up new potential for assessment of the effects of therapeutically targeting microglia.
This is only one of several types of molecular targets that should help us to understand the pathologic findings of this disease, however. PET has an increasing potential for defining pathologic findings in a specific neuronal population, which may allow differentiation of individual neuronal subclasses based on such features as neurotransmitter receptor distribution. Histopathologic evidence has suggested selective neuronal vulnerability in MS; smaller axons seem to be more susceptible to damage. The hypothesis that GABAergic signaling is relatively selectively reduced in cortical lesions of MS can be tested directly using receptor probes, such as flumazenil. Information related to this could be used to suggest modulatory neurotransmitter treatments that might modify symptoms or, if PET measures were extended to stem cell–specific markers, the integration of stem cells after transplantation.
Direct myelin imaging is a goal that should be achievable in the short term. Congo red–based molecules bind to myelin with a relative specificity that suggests their possible use as myelin PET tracers. An initial set of studies has shown practicality for in vivo assessment and good specificity and sensitivity to MS-associated demyelination in vitro. What is needed now is further characterization of binding sites to allow accurate interpretation of changes in binding potential. It should be possible to develop tracers directed against more than one myelin component to get better insight into myelin structure-function changes with MS. Absolute quantitation of the PET signal is possible. Although the PET experiment is always going to be more expensive and invasive than MR imaging, this offers a way of “calibrating” indirect MR imaging measures in smaller experimental medicine studies.
A new era of imaging may be heralded by imaging agents that are developed in parallel with innovative therapies and have the potential to answer critical questions related to the proof of pharmacology (eg, labeled stabilized antisense oligonucleotides that could be used to assess delivery and half-life of the cognate therapeutic molecules). Potentially therapeutic antibody fragments (eg, domain antibodies) can be labeled with a variety of positron-emitting isotopes and have kinetic characteristics favorable to imaging. Because of the exquisite selectivity with which they can be targeted and their high affinity, these and similar antibody-related molecules may provide ideal ways of labeling individual cell populations. Although substantial technical hurdles remain, this would provide a way of “tagging” cells to study their trafficking and their longer term distribution in the body. This would enable studies evaluating those immune-modulatory agents directed against the ways in which individual cell populations migrate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree