© American Academy of Pain Medicine 2015
Timothy R. Deer, Michael S. Leong, Asokumar Buvanendran, Philip S. Kim and Sunil J. Panchal (eds.)Treatment of Chronic Pain by Interventional Approaches10.1007/978-1-4939-1824-9_4040. Brain Stimulation for Pain
(1)
Department of Neurosurgery, University of Illinois at Chicago, 912 South Wood Street, M/C 799, Chicago, 60605, IL, USA
Abbreviations
CCH
Chronic cluster headaches
CT
Computed tomography
DBS
Deep brain stimulation
DREZ
Dorsal root entry zone
ET
Essential tremor
FDA
US Food and Drug Administration
MCS
Motor cortex stimulation
MEG
Magnetoencephalography
MER
Microelectrode recording
MRI
Magnetic resonance imaging
OCD
Obsessive-compulsive disorder
PAG
Periaqueductal gray area
PD
Parkinson disease
PET
Positron-emission tomography
PNS
Peripheral nerve stimulation
PVG
Periventricular gray area
SCS
Spinal cord stimulation
STN
Subthalamic nucleus
TRD
Treatment-resistant major depression
VPL
Ventroposterolateral nucleus of thalamus
VPM
Ventroposteromedial nucleus of thalamus
Key Points
Deep brain stimulation (DBS) in treatment of pain has a long and fascinating history that includes a decade and a half of its widespread use for variety of indications in the mid-1970s to the late 1980s.
Use of DBS for pain greatly diminished after its approval for this indication was rescinded by FDA due to lack of conclusive evidence regarding its effectiveness.
Most commonly used targets for DBS in treatment of pain include sensory nuclei of thalamus (primarily, ventroposteromedial and ventroposterolateral nuclei) and “central gray” matter (periaqueductal and periventricular gray areas).
Currently, DBS is being explored as an option for otherwise refractory chronic cluster headaches, and the initial clinical results are quite encouraging.
Introduction
In the ever-advancing world of neuromodulation, use of electrical stimulation for pain relief has been a dominant theme – mainly due to wide acceptance of spinal cord stimulation (SCS) over the last four decades and, more recently, with rebirth of peripheral nerve stimulation (PNS) approach. The relative simplicity of these interventions resulted in a shift among practitioners who use it – and if in the beginning most neuromodulation procedures were done by neurosurgeons, vast majority of both SCS and PNS systems are now implanted by non-surgeons, primarily anesthesiologists and physiatrists who specialize in the field of pain medicine. Even those interventions that in the past required neurosurgical expertise – laminectomy for insertion of SCS paddles and nerve explorations for placement of PNS electrodes – are now frequently done by our orthopedic and plastic surgery colleagues.
The only target of neuromodulation where neurosurgeons proudly keep their surgical monopoly is the brain. And when it comes to indications for cerebral neuromodulation procedures, one immediately thinks of movement disorders, Parkinson disease (PD), essential tremor (ET), and dystonia, all of which have been successfully treated with stimulation of thalamic nuclei or basal ganglia. More recently, this approach of electrical stimulation of deep cerebral structures – both in the white and gray matter, usually referred to as deep brain stimulation (DBS) – has been used in treatment of psychiatric conditions, primarily obsessive-compulsive disorder (OCD) and treatment-resistant major depression (TRD); mixed motor and behavioral disorders, such as Tourette syndrome; and refractory epilepsy. Gradually, DBS has become a standard in surgical treatment of some of these conditions – essentially replacing destructive interventions that were commonly used in the past.
In addition to stimulation of deep cerebral structures, surgical neuromodulation may also target surface of cerebral convexity. This approach, through either epidural or subdural electrodes, is referred to as cortical stimulation. While cortical stimulation has been tried for treatment of tinnitus, depression, poststroke weakness, tremor, and Parkinson disease, one of the best known indications is chronic neuropathic pain for which the contralateral motor cortex is stimulated with implanted electrode. Motor cortex stimulation (MCS) for treatment of pain is a subject of separate chapter in this book. Here, we focus on DBS procedures and their applications in treatment of chronic pain.
DBS for pain has long and fascinating history [27, 40, 69]. First mentions of electrical stimulation suppressing pain sensation came from laboratory animals in the 1950s [51, 61], and right around that time, first clinical experience in humans was reported by Heath [29, 30] and Pool et al. [57] when they investigated brain activity in variety of subjects. Soon thereafter, multiple publications described stimulation of deep cerebral structures in patients with different clinical conditions [22, 47, 75], primarily with cancer pain and other refractory pain syndromes.
Instead of discussing different mechanisms of action that have been proposed to explain DBS effects in chronic pain, we will briefly go over the targets for DBS interventions, some basic procedural details, and the reasons why DBS is rarely utilized in contemporary clinical practice. Interested readers may gather more in-depth information from multiple recently published reviews [12–14, 25–27, 36, 40, 53, 69] as with decline in number of DBS procedures worldwide and almost complete abandonment of this approach in the United States, detailed literature reviews seem to outnumber original case series.
Targets for Deep Brain Stimulation in Treatment of Pain
Over the several decades since DBS was introduced, multiple targets have been explored – frequently with very encouraging initial results. Published reports concentrated on various distinct cerebral regions that represent different components of the pain-processing system including sensory pathways of the midbrain and their relays in thalamus, parts of the limbic system, and connections between the sensory and limbic areas.
Among the most commonly used targets are lemniscal system and thalamic nuclei that were explored as early as the late 1960s due to their known involvement in the processing of somatic pain [31, 45, 46, 50, 65]. Around the same time, the septal area [22, 66] and the internal capsule [3, 18] were successfully tried for pain control. Somatotopic organization of the posterior thalamic nuclei allowed one to selectively stimulate those parts that correlated with location of pain [73]. Production of paresthesias in the region of pain distribution supported use of thalamic DBS in cases of neuropathic pain, with medial locations (ventroposteromedial (VPM) nucleus) used in treatment of facial pain [31] and lateral locations (ventroposterolateral (VPL) nucleus) used for treatment of pain in the extremities [45]. Similar to other neuromodulation approaches (SCS and PNS), VPM and VPL DBS elicit paresthesias in the contralateral face or body areas, and as long as the paresthesia location matches location of the neuropathic pain, the pain relief is expected. In addition to that, medial thalamic nuclei were stimulated due to known involvement of this part of the thalamus in pain and emotional processing [8, 65, 72].
Although this approach was effectively used for neuropathic pain and associated phenomena, it was less effective for truly nociceptive pain conditions. For this group of indications, stimulation of periaqueductal gray matter (PAG) and periventricular gray matter (PVG) was suggested and tried. Since PAG and PVG (so-called central gray) are involved in descending modulation of pain, it is indeed conceivable that stimulation of this region would suppress nociception and produce pain relief through either monoaminergic [4] or opioid-mediated pathways [63]. The opioid hypothesis was supported by finding both endorphin-like [6] and enkephalin-like [7] substances in ventricular cerebrospinal fluid during PAG/PVG stimulation and by reversal of analgesia that it produced with administration of naloxone [2, 5, 32]. However, since these findings were rather nonspecific and not consistent, it was concluded that the mode of DBS action in generation and suppression of chronic pain is so far unknown [33, 48, 76].
Finally, the last target from the traditional era of DBS for pain (before its approval was rescinded by US Food and Drug Administration (FDA)) was the medial parabrachial area of the rostral dorsolateral pons, particularly the Kölliker-Fuse nucleus [78]. Stimulation of this area was reserved for the patients who failed to improve or improved only temporarily with either thalamic or PVG/PAG DBS procedures.
The aforementioned decision of FDA to rescind approval of DBS for treatment of pain was based on less-than optimal results of two multicenter studies [13]. These studies were put together by Medtronic (Minneapolis, MN), the sole manufacturer of DBS equipment at that time, and failed to reach expected endpoints thereby negating positive findings reported in multiple large series published in the 1970s, 1980s, and 1990s [17, 26, 33, 35, 36, 41, 49, 50, 56, 60, 63, 64, 71, 77]. Nevertheless, the clinical experience continued to accumulate with several enthusiastic centers worldwide although most recent series came from outside of the United States [16, 28, 34, 53, 58, 59]. In addition to general series dealing with nonuniform cohorts of patients with neuropathic and nociceptive pain, there are now dedicated reports on use of DBS in treatment of poststroke pain [52], trigeminal postherpetic neuralgia [23], neuropathic pain in head and face [24], phantom limb pain [11], and pain due to spinal cord injury [58].
Low efficacy of DBS for pain and loss of its regulatory approval were not the only reasons for its gradual decline. The other treatment modalities such as improved nonsurgical means of pain control, more versatile spinal cord stimulation devices, and introduction of intrathecal opioids provided better choices for the patients and clinicians. In addition to that, not-so-low rate of complications made the entire modality less attractive, particularly when it is used exclusively on “off-label” status.
The concept of DBS for pain was reborn with discovery of discrete activation in hypothalamus during attacks of cluster headaches in 1998 [43]. Followed by discovery of similar pattern in patients with other painful conditions that involve face and head, such as short-lasting unilateral neuralgiform headaches with conjunctival injection and tearing, hemicrania continua, and paroxysmal hemicranias [42], a group of neurosurgeons implanted DBS into ipsilateral hypothalamus of a chronic cluster headache (CCH) patient [37]. This was followed by experience with bilateral implantation in a patient with bilateral symptoms [38] and then by several series of patients with intractable CCH [10, 19–21, 39, 67, 70] as well as other headache syndromes [9, 74]. Although the results were far from uniform with documented failure in some patients [55] – if anything, very similar to the past experience with DBS for other pain syndromes – and there was a mortality reported from DBS procedure in this otherwise nonfatal condition [67], hypothalamic DBS remains perhaps the most promising DBS application today, mainly due to severe disability and relative refractoriness of CCH and hemicranias to conventional, less invasive treatments.
Deep Brain Stimulation Procedure
Technically, DBS for pain uses very similar – or even identical – approach to DBS for movement disorders (PD and ET). The surgery usually consists of two parts with the first one done under local anesthesia and the second under general anesthesia. Stereotactic approach is used for implantation of DBS electrodes. The coordinates for stimulation target(s) are calculated based on MRI of the brain that is obtained prior to the surgery itself. Even though frameless DBS is gaining popularity, most centers continue using frame-based approaches for DBS when it comes to pain indications.
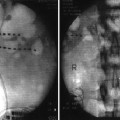
This means that the surgery starts with application of stereotactic frame. This is a metal contraption that is rigidly attached to the patient’s head with several sharp pins. The frame serves as a reference for stereotactic coordinates and as a base for stereotactic surgical instruments. The pin insertion sites are anesthetized with local anesthetic, and then, once the frame is secured, the patient undergoes high-resolution stereotactic imaging, which may be either a brain MRI or a combination of CT scan with either MRI or ventriculography. In the past, ventriculography was the gold standard imaging modality, but it became replaced by CT and then by MRI as technology advanced. Currently, ventriculography is considered in surgical targeting for those patients who cannot have MRI and is used very rarely. The MRI may be done when the frame is already attached, or, alternatively, it may be obtained before the day of surgery and then “fused” with stereotactic CT of the brain. However, since the resolution of current MRI systems does not allow direct visualization of thalamic nuclei, the surgical planning is done based on the atlas coordinates that are referenced against classic landmarks. These landmarks include anterior and posterior commissures, height of the thalamus, and third ventricular width, all of which have been in use since the time of ventriculography. This atlas-based approach is expected to change once higher power MRI systems become available for clinical use as preliminary data from 7 T imaging indicate that direct visualization of thalamic nuclei is indeed feasible [1], similar to the change in DBS practice since the introduction of 3 T MRI allowed better direct visualization of subthalamic nucleus (STN) [68].
Once the imaging and subsequent surgical planning are completed, the electrodes are implanted using a standard approach, usually from pre-coronal burr hole, with trajectory planned in such a way that blood vessels and ventricles are avoided. Physiological confirmation of target correctness may include microelectrode recording (MER) depending on the target and the surgeon’s preference, but must include macrostimulation in order to determine thresholds for desired effects and for side effects.
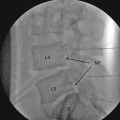
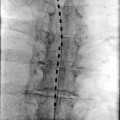
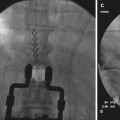
Once the physiological testing is completed, the DBS electrode gets inserted into desired location, and its position is routinely confirmed with intraoperative fluoroscopy and postoperative CT scan. The hardware for DBS is beyond the scope of this chapter – but it is worth mentioning that the originally used DBS electrode with four separate stimulating contacts (model 3380, Medtronic, Minneapolis, MN) was discontinued in the early 1990s. The next model of DBS electrode (3387) was eventually approved by FDA for ET and PD treatment. With this approval, DBS electrodes remain available for other indications such as treatment of pain but only on an “off-label” basis [13].
DBS electrodes that are implanted into desired location are secured to the skull with cement, metal plates, or special locking devices. For the purposes of stimulation trial, a temporary extension cable is then connected to each electrode and tunneled under skin to a distant exit site. During this trial, the patient and surgeon determine whether DBS results in expected improvement and if there are any side effects that would prevent long-term DBS use. In case of implantation of multiple electrodes, a decision is made whether all of them are needed for best clinical effects. Following this trial that usually lasts a week or so, the implanted electrodes are either removed if the trial fails or get internalized if the trial succeeds. Internalization is performed under general anesthesia, and the permanent extension cables are tunneled toward the generator site which usually gets implanted in the infraclavicular region.
Postoperative DBS Management
The programming of DBS devices implanted for treatment of pain is similar to other neuromodulation applications, such as SCS for pain and DBS for movement disorders. The parameters of stimulation greatly depend on location of the DBS electrode contacts. In the early days, these electrodes were placed either in the thalamic nuclei, in the internal capsule, or in the central gray areas (PAG/PVG). More recently, it has become a common practice to put electrodes into both thalamic nuclei and central gray and then decide which one of them will be internalized or whether both areas need to be stimulated for optimal pain relief. The choice of the best contacts and stimulation parameters is determined by degree of pain relief, but since it may not occur immediately, attention is paid to location of paresthesias, particularly in the case of thalamic sensory nuclei, and to presence and tolerability of side effects that may be quite pronounced. At the same time, there is certain “insertional effect” where almost a half of patients in one series had a substantial improvement in pain severity in the absence of stimulation [28]. This may necessitate certain delay in the beginning of active programming following device implantation.
Most common side effects of DBS for pain are relatively minor. The transient headache occurs in more than half of all patients [36]. In addition to this, there are multiple issues related to the location of the electrodes – the proximity of PAG/PVG to oculomotor centers explains complaints related to double vision, blurred vision, and oscillation of objects in the visual field (oscillopsia), as well as sensation of nausea. Objectively, these patients may present with nystagmus and gaze palsy. Most of these phenomena, however, are transient and short-lasting. The stimulation-related sense of impending doom and severe apprehension are sometimes observed in PAG stimulation therefore limiting the patient’s willingness to use the device [77




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree