Peripheral nerves can be categorized based on their conduction velocity and diameter (Table 38.1).
Description of nerve fibers | Group | Diameter (μm) | Conduction velocity (m/s) | |
|---|---|---|---|---|
Myelinated somatic | Alpha α | 20 | 120 | |
Beta β | ||||
A | Gamma γ | S-40 (pain fibers) | ||
Delta ∂ | 3–4 | S-40 (pain fibers) | ||
Epsilone | 2 | 5 | ||
Myelinated visceral (preganglionic autonomic) | B | <3 | 3–15 | |
Unmyelinated somatic | C | <2 | 0.5–2 (pain fibers) | |
The aforementioned nerve layers and inconsistent fascicular topographic arrangement provide an anatomic explanation not only for the impendence to overcome but also the cumulative effect of cathodal stimulation employed in peripheral neuromodulation [14]. PNS and PNFS directly inhibit primary nociceptive afferents and suggest central sensitization can be subverted by peripheral nociceptive suppression. Moreover, percutaneous PNS and PNFS approaches utilize devices that were designed for use in the epidural space, and therefore peripheral use is accompanied by frequent, although minor, complications.
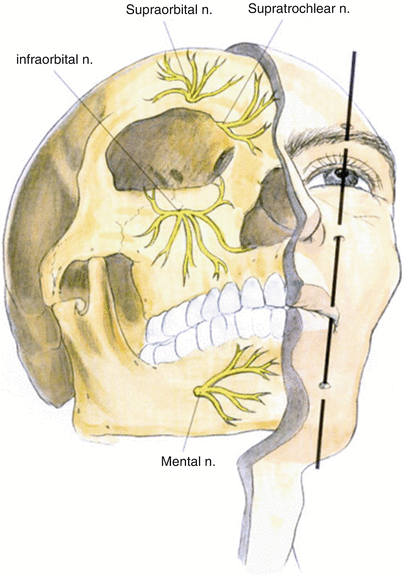
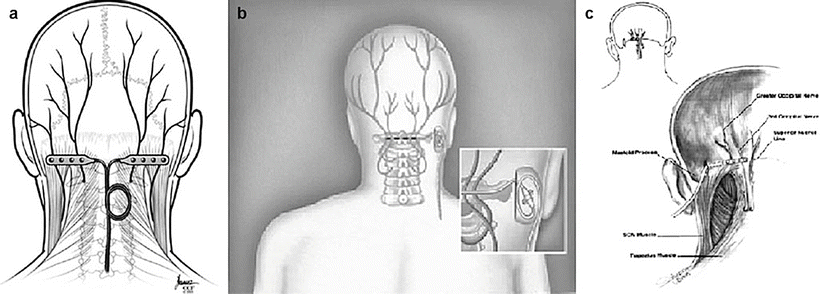
Image guidance is recommended to perform percutaneous PNS. Unlike the spine, the use of imaging in the periphery is complex and is impacted by patient positioning, variation in bony landmarks, and obesity. Consequently, neuropathic pain peripheral nerve targets are only limited by the ability to visualize them, either directly or indirectly (by anatomic correlation to well-defined osteal landmarks or using US guidance and/or fluoroscopy). Common sites include the supraorbital, the infraorbital, and the greater occipital nerves in the head and neck; the ulnar, median, and suprascapular nerves in the upper extremity; the intercostal, ilioinguinal, iliohypogastric, and genitofemoral nerves in the trunk; and the lateral femoral cutaneous, saphenous, sciatic, and posterior tibial nerves in the lower extremity.
Clinical Examples
PNS and PNFS trialing and permanent implantation require a meticulous sterile preparation and wide enough operative fields to visualize the necessary surgical targets. Further, peripheral nerve stimulation is only limited by the ability to visualize the target nerve and IPG implantation location. Peripheral nerve stimulation targets will be discussed separately.
Trigeminal Peripheral Nerve Stimulation
Infraorbital Nerve Stimulation Trial
The infraorbital nerve is one of the terminal branches of the maxillary division of the trigeminal nerve and exits via the infraorbital canal (please refer to Fig. 38.3).
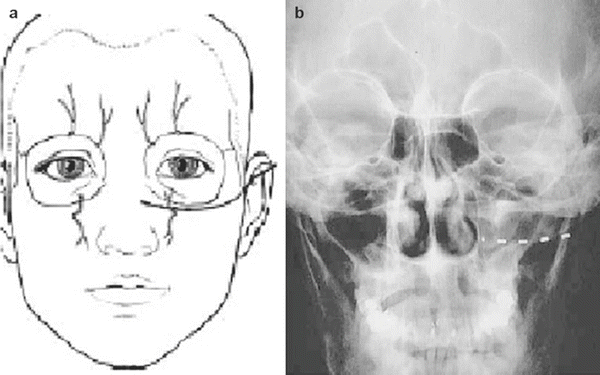
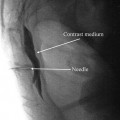
The patient is positioned supine, prepped, and draped in sterile fashion (alcohol should be avoided in the face to avoid corneal irritation). Fluoroscopy is used in the anterior-posterior view to approximate the target. The target site is the infraorbital foramen on fluoroscopy. If it cannot be appreciated, the lead is placed approximately 1 cm below the orbit and just lateral to the ipsilateral nose, as described by Slavin et al. [16]. The entry point is lateral and inferior to the eye over the zygoma. Again, after judicious local anesthetic use at the entry point, a bent introducer needle to accommodate the contour of the face is inserted and directed to the target zone under fluoroscopy. Once the needle is in the correct position, the percutaneous cylindrical lead is introduced with care not to direct the distal tip of the needle too superficial to avoid lead tip erosion. The introducer needle is withdrawn slightly to allow intraoperative testing (Fig. 38.3).
Once therapeutic stimulation is achieved, the needle and stylet are removed, leaving the lead in place. After serial imaging to confirm placement, the lead is sutured in place, and a sterile dressing is applied and taken to the recovery area.
Supraorbital Nerve Stimulation Trial
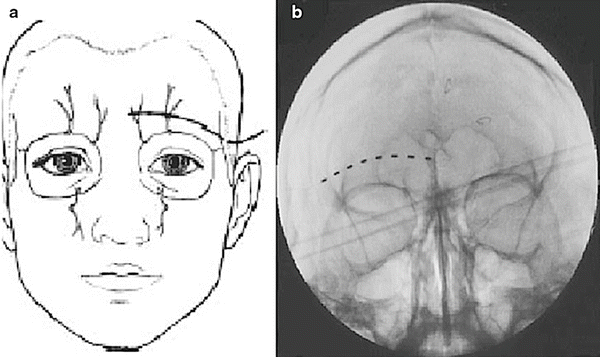
Slavin et al. described the most commonly employed technique for terminal branch trigeminal nerve stimulation [16, 17]. The patient is positioned and prepared as discussed for the infraorbital nerve stimulation. Fluoroscopy is used in the anterior-posterior view to approximate the target. A skin wheel is raised using 1 % lidocaine and is raised approximately 3–4 cm lateral to the lateral corner of the eye. An incision is then made, where a standard 14 G Tuohy needle (bent to allow and follow the contour of the face), is directed toward the midline approximately 1 cm above the supraorbital ridge until it is approximately 1 cm from the midline (Fig. 38.4). Avoiding too superficial trajectory will avoid lead tip erosion.
The stylet is removed, the percutaneous electrode is placed, and the needle is withdrawn to allow for intraoperative stimulation testing. Judicious use of local anesthetic at the puncture site will allow for intraoperative testing.
Once the desired therapeutic paresthesia overlying the patient’s pain is achieved, the needle is withdrawn and removed while performing serial fluoroscopic guidance to ensure no inadvertent lead migration. The externalized lead is then secured with the supplied plastic anchor of the surgeon’s choosing and nonabsorbable sutures. A sterile dressing is applied and the patient is taken to the recovery area.
Supra- and Infraorbital Nerve Permanent Implant
For the permanent implantation of both the infra- and supraorbital leads and IPG, general anesthesia is recommended (with laryngeal mask airway if feasible). The patient is again positioned supine with a slight contralateral head turn to provide access to the retroauricular location. A meticulous sterile prep and drape is required to accommodate tunneling and IPG site location. Commonly, the infraclavicular sight is chosen.
The permanent percutaneous lead is inserted as described for the trial. An additional incision is made in the ipsilateral retroauricular location after appropriate topicalization. Two techniques have been described for tunneling the lead’s IPG connection portion. One is simply using the introducer needle with stylet in place (bent to accommodate the contour of the tunneling from the retroauricular incision to the anterior incision). The stylet is then removed, the lead introduced, and the needle withdrawn, leaving the tunneled lead.
From the retroauricular location, the lead is secured with the supplied plastic anchor using nonabsorbable suture. A stress loop is recommended (approximately 2–3 cm in diameter), and the lead is attached to the extension cable. It is recommended to place the extension cable connection in close approximation to the retroauricular incision to allow for easy access if reoperation is required.
IPG site location is largely the surgeon’s preference [19]. In tunneling to infraclavicular and periscapular, one must remain in the posterior triangle of the neck and avoid the suprascapular nerve. Tremendous care is needed to avoid the external jugular which is adjacent to and superficial to the sternocleidomastoid muscle. Specifically, one must recognize the mobility of neck and shoulder especially if placing IPG in infraclavicular and periscapular. Measurement of length of extensions and electrodes is necessary to accommodate the flexion and extension of the neck. Avoidance of placement around osteal structures may reduce pain overlying the IPG device.
Regardless of IPG location, careful dissection (avoiding excessive blunt dissection), meticulous hemostasis, and anchoring to the perimuscular fascia are crucial to avoid IPG dislodgement. Anchoring to the perimuscular fascia is crucial and requires the use of tiny and soft or suture anchors given the limited subcutaneous tissue present.
Copious non-pressurized irrigation is performed at all incision sites, and layered closure is performed with absorbable suture. It is recommended to avoid placing sutures overlying the IPG, as this may impair wound healing and increase the chance of wound dehiscence. Sterile dressing is applied and the patient is recovered in the postoperative area.
Greater Occipital Nerve Stimulation Trial
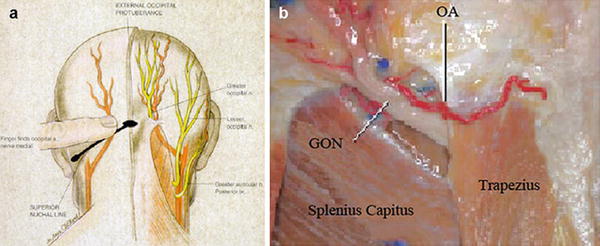
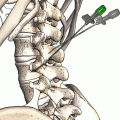
The greater occipital nerve is also known as the second occipital nerve and is the medial branch of dorsal primary rami of C2. After discovery of the trigeminocervical complex, the greater occipital nerve has become a popular target for the treatment of headache [20–22]. Anatomic dissection characterizes the location from osteal landmarks, including the mastoid process and the occipital protuberance [23–25]. (It is generally found 1.4–1.6 cm lateral from the external occipital protuberance and 2.91–3.7 cm inferior). The nerve is consistently medial to the occipital artery (see Fig. 38.5).
There are multiple techniques described to stimulate the greater occipital nerve, with major differences centering on target location (C1–2 vs. nuchal ridge/retromastoid), lead trajectory orientation (medial to lateral or lateral to medial), and type (percutaneous cylindrical vs. paddle) (Fig. 38.6) [17, 26, 27].
Variation in location and lead type implantation for occipital nerve stimulation qualifies the initial high migration rates and aberrant muscular stimulation with percutaneous leads [17, 22, 26]. Proponents of paddle leads argue that less migration may occur because of the larger surface area of the lead and unidirectional current [27]. Muscle spasms of splenius capitis may be subverted by placement of the electrodes at or above the nuchal line, as opposed to the C1–2 level, where too superficial of a lead placement may increase the chance of erosion and burning sensations, while too deep a placement may cause aberrant muscular stimulation.
The trial is performed with the patient in the prone position. The surgical site is prepared by hair and meticulous sterile prep and drape in the normal fashion, leaving the entry site exposed. Image guidance is a prerequisite; fluoroscopy is commonly employed. After the target location is chosen, the incision site is identified. Care must be taken not to anesthetize the greater occipital nerve, and therefore judicious local anesthetic should be used at the incision site using 1 % lidocaine. The medial, nuchal line approach will be described here in further detail (Fig. 38.7).
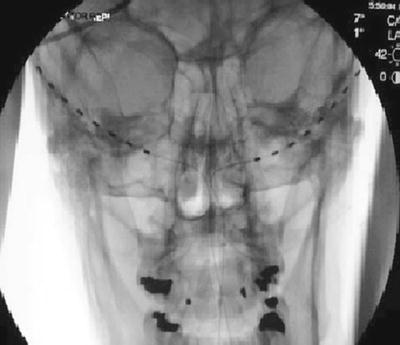

Fig. 38.7
Percutaneous cylindrical lead placement in AP fluoroscopic projection
An incision is made in the midline just caudal to the occipital protuberance. The needle is bent to accommodate the contour of the head. Under fluoroscopic guidance, lead is placed along the nuchal ridge ipsilateral to the target greater occipital nerve. Once appropriate lead position is achieved, the needle is withdrawn slightly to allow for intraoperative stimulation testing. Once therapeutic stimulation is achieved, the needle is removed and the lead is secured using the plastic anchor provided and nonabsorbable suture. A sterile dressing is applied, and the patient is further recovered and programmed in the recovery room.
Greater Occipital Nerve Permanent Implant
Preparation and anesthesia and the lead placement procedure are largely the same for the occipital nerve trial and implant. The surgical prep site is extended to a larger area, however, to accommodate the IPG location. As discussed previously, IPG location and migration rates have been compared with superior outcomes suggested by infraclavicular and abdominal locations versus periscapular and gluteal sites, respectively. Like SCS, strain loops are created at the incision site by careful lateral dissection. Plastic anchors and nonabsorbable sutures are used to suture the lead to the dorsal fascia. A third incision is made and carefully dissected to accommodate the IPG. If extensions are needed and tunneling is required over a great distance, additional incisions with sequential tunneling may be required. Irrigation is performed at all incision sites, and layered closure is recommended, again, with care not to create a suture line overlying the implanted device. Sterile dressings are applied and further programming is performed in the recovery area.
Ulnar Nerve Stimulation Trial
The ulnar nerve is the most caudal portion of the brachial plexus, arising from the medial cord with nerve roots originating at C8–T1. The nerve descends medially to the brachial artery in the proximal arm, anterior to the medial triceps of the triceps, and at the elbow, it resides in the grove of the medial epicondyle.
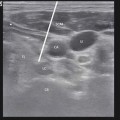
Patient is positioned supine and patient preparation, including sterile prep and preoperative antibiotics, is performed in the usual manner. As described by Huntoon et al. [28] from the reliable and easily identified ulnar nerve location at the medial epicondyle, the nerve is traced in the axial sonographic view to approximately 9–13 cm proximal. Once the nerve is located, a skin wheel is raised with lidocaine 1 % and a skin nick is created. Under a live axial view of the ulnar nerve, the needle is then introduced via the long axis of the probe, placing the lead deep, adjacent, and perpendicular to the ulnar nerve. The needle is retracted and stimulation testing commenced. Anchoring of the lead to the skin was performed using the plastic anchors and nonabsorbable suture (Fig. 38.8




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree