Stage
Clinical features
I
Arteriosclerosis with no symptoms
II
Intermittent claudication with no symptoms at rest
IIa
Intermittent medium claudication (after 200 m of walking)
IIb
Intermittent severe claudication (before 200 m of walking)
III
Claudication, symptoms at rest and night pain without tissue involvement
IV
Grade III + tissue loss (ischemic ulceration; gangrene)
IVa
With local inflammation
IVb
With widespread inflammation
Exclusion Criteria (Adapted from [26])
1.
Life expectancy <3 months.
2.
Lack of patient compliance.
3.
Ischemic ulcerations >3 cm.
4.
Wet gangrene.
5.
Presence of infection.
6.
Imminent acute obliteration requiring emergency amputation.
7.
The use of SCS in the presence of an on-demand pacemaker is relatively contraindicated.
8.
MRI with body coil is absolutely contraindicated after SCS implantation.
Mechanism of Action
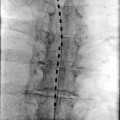
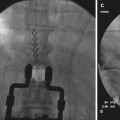
Ischemic pain is the only nociceptive pain that is proven to respond to SCS. As opposed to mechanisms involved in neuropathic pain attenuation, beneficial effect of SCS on ischemic pain involves attenuation of tissue ischemia as a result of increasing and/or redistributing blood flow to ischemic area or by decreasing tissue oxygen demand to the ischemic area. SCS has been shown to improve microcirculation in an animal model [27]. These effects appear to be mediated by two mechanisms: (1) suppression of efferent sympathetic activity (via nicotinic ganglionic receptors and mainly alpha-1 adrenoreceptors in the periphery) and (2) antidromic mechanisms involving the dorsal roots that stimulate the release of calcitonin gene-related peptide (CGRP) [15]. The balance of these mechanisms depends on a variety of factors including the tone of the sympathetic nervous system, patient factors (diet, genetic differences, etc.), and intensity of SCS [29]. Figure 45.1 illustrates the mechanisms and neurotransmitters known or hypothesized to be involved in the effects of SCS in PAOD. A thorough review of the putative mechanisms behind the effects of SCS on peripheral vascular disease is found in Wu et al. [29].
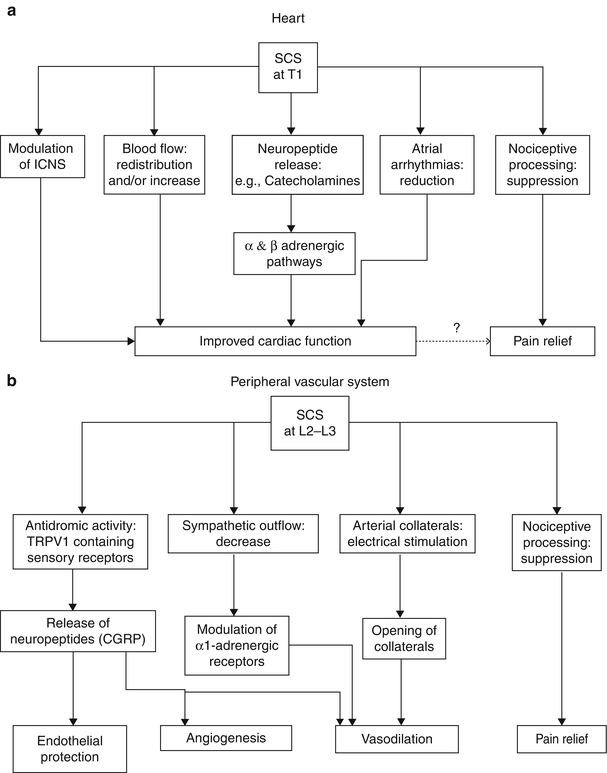

Fig. 45.1
(a, b) Illustrates the mechanisms and neurotransmitters known or hypothesized to be involved in the effects of SCS in PAOD
Spinal Cord Stimulation for Angina Pectoris
Angina usually occurs during episodes of vasospasm or occlusion of the coronary vessels that results in an imbalance between the supply and the demand of oxygen in the heart due to decreased blood flow to the heart. Patients with angina pectoris are often managed effectively through pharmacological treatment with beta-receptor blocking agents, long-acting nitrates, and calcium antagonists, by revascularization procedures such as coronary bypass surgery (CABG) or by percutaneous transluminal coronary angioplasty (PTCA); however, a segment of patients suffering from chronic angina pectoris does not respond to conventional treatments [30]. Many patients suffering from severe disabling angina (New York Heart Association (NYHA) classes III–IV) suffer from concurrent comorbidities, making them unsuitable for major invasive procedure. Other patients suffer from widespread obliteration or distal lesions that do not permit successful surgical interventions. Regardless of the etiology, these patients suffer from what is termed treatment refractory angina and have a low quality of life, limited physical capacity, and frequent hospital admissions representing a large costs for society [31]. Patients suffering from treatment refractory angina led clinicians to develop alternative strategies such as neuromodulation to provide pain relief. In European estimates, refractory angina is a common condition with a prevalence of 100,000 patients [32]. SCS has been used to treat such therapy-resistant angina pectoris since the mid-1980s [33, 34]. The first ten cases of SCS directed specifically at the treatment of angina were reported in 1987 by Murphy and Giles [34].
Patient selection involves specific criteria that maximize the likelihood of clinical success and eliminates patients that are not likely to benefit in an effort to prevent these patients from undergoing an unnecessary procedure in lieu of their multiple comorbidities. The following inclusion and exclusion criteria are adapted from [26].
Inclusion Criteria
1.
Severe angina pectoris (NYHA classes III–IV or Canadian Cardiovascular Society classifications I–IV) refractory to conventional treatment. Patients who have been subjected to thoracotomy may present with post-thoracotomy syndrome or intercostal neuralgia, so a careful pain assessment is mandatory. Efficacy of treatment is related to angina that is due to a reversible cardiac insult.
2.
Significant coronary artery disease.
3.
Demonstrated reversible myocardial ischemia as a cause of patient’s symptoms.
4.
Patients diagnosed as suffering from syndrome X.
5.
Success of TENS in pain alleviation indicates a high likeliness for a positive response to SCS.
Exclusion Criteria
1.
Acute myocardial infarction.
2.
Presence of other ongoing heart diseases such as pericarditis or myocarditis.
3.
Presence of on-demand pacemaker (relative contra-indication).
4.
MRI investigation with body coil is an absolute contraindication after implantation.
Mechanism of Action
Ischemic heart disease often presents as shortness of breath and angina pectoris, described clinically as an extremely intense substernal crushing pain and that usually radiates to the chest, shoulder and left arm, and occasionally to the neck and jaw [33, 35]. This nociceptive information is transmitted by sensory afferent fibers that enter the C7–T5 spinal segments and synapse on spinothalamic tract cells, and cells of other ascending pathways, that also receive converging cutaneous and muscle input from the overlying somatic structures such as the chest and upper arm [35]. This nociceptive information is also transmitted in nociceptive vagal afferent fibers that converge on spinothalamic tract cells in the upper cervical segments that also receive somatic convergent input from the neck and jaw [35].
Although investigators disagree about the mechanisms responsible for the alleviation of angina pain by SCS, human studies have shown that SCS reduces ischemia by redistributing coronary blood flow [36, 37] and also by decreasing cardiac myocyte oxygen demand [38]. Patients also benefit from increased time to angina in exercise tests [37], increased resistance to critical ischemia [38], and modulation of cardiac neurons leading to decreased dysrhythmias [14, 39]. Of particular importance is that SCS does not mask myocardial infarction [40, 41]. Animal studies have shown that SCS suppresses pain transmission in nociceptive pathways and improves cardiac function by stabilizing neurons of the intrinsic cardiac nervous system, reducing infarct size via adrenoreceptors, and reducing ST segment changes during ischemic episodes, and reducing atrial arrhythmias [42] (for review see Foreman et al. [43]; Wu et al. [29]). Figure 45.1 illustrates the mechanisms and neurotransmitters known or hypothesized to be involved in the effects of SCS in angina.
Preoperative Considerations
Careful preoperative evaluation of patients with PAOD and angina pectoris is mandatory since these patients often have an assortment of coexisting comorbidities that makes even a minimally invasive procedure a challenge. SCS requires active patient participation to ensure proper placement of electrodes with the goal of covering the patient’s pain with a comfortable level of paresthesia that completely covers the affected area. During preoperative evaluation, a psychological evaluation performed by a psychologist or a pain-oriented psychiatrist may reveal certain psychological conditions that may exclude patient’s from SCS treatment including major personality disorders, deficient capacity to collaborate and to communicate their pain, and drug-seeking behavior or abuse. A thorough analysis of the patient’s pain is mandatory since many mixed pain conditions (i.e., coexisting neuropathic and nociceptive pains) may not respond to treatment and may result in clinical failure. Risks and benefits including spinal cord or nerve injury, dural puncture, epidural hematoma, headache, and infection should be discussed with the patient, and all questions and concerns should be answered or addressed appropriately.
Patients with chronic pain conditions are usually being treated with multiple pain medications, and in general, all pain medications should be continued until 2 h before the procedure is performed. Transdermal patches may be continued throughout the procedure. A majority of chronic pain patients do not respond well to increased or additional pain and are seemingly immune to normal doses of systemic analgesics. Caution should be used with liberal dosing of opioids as these patients remain vulnerable to overdose and oversedation may lead to difficulty in communicating with patient to ensure proper electrode placement. Copious use of local anesthetic may be utilized; however, the maximum dose of local anesthetic should be calculated preemptively to avoid toxic dosages. Patients on antipsychotic medications are at risk for developing neuroleptic malignant syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree