Brain Tumors: Other Primary Tumors, Metastases, and Radiation Injury Imaged with PET and SPECT
Ian Law
Lise Borgwardt
Liselotte Højgaard
Approximately half of all malignancies in the brain are gliomas, and the other half are metastases, primarily intracranial lymphomas and nongliomatous primary brain tumors, the most frequent ones being meningiomas, pituitary adenomas, and schwannomas. Smaller studies have evaluated the use of PET in patients with intracranial metastases and primary intracerebral lymphoma in acquired immunodeficiency syndrome (AIDS) patients, but no extensive systematic series are available, including none with image coregistration between PET and CT or PET and MRI.
Fluorodeoxyglucose PET (FDG-PET) lacks sensitivity for the detection of brain metastases, as metastases may show both hyper- and hypometabolism of FDG, even among metastases in the same patient. Other and new radiopharmaceuticals such as carbon 11 (11C) methionine (MET), [18F]methyltyrosine or ethyltyrosine, and 11C- or 18F-labeled thymidine or choline may be useful, especially in combination with MRI or CT in individual patients in whom precise delineation of metastases is mandatory before stereotactic therapy.
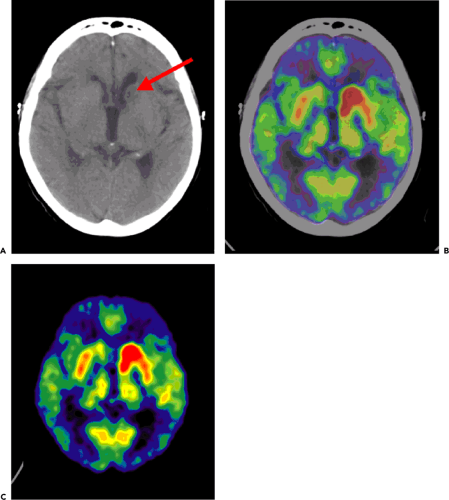
In AIDS patients, FDG-PET has the ability to discriminate between primary central nervous system (CNS) lymphoma (Fig. 23.1) with high uptake and infectious lesions with lower FDG uptake. For primary CNS lymphoma in non-AIDS patients, brain FDG-PET seems useful in combination with MRI or CT for stereotactic biopsies. An alternative is thallium 201 (201Tl) SPECT.
Nongliomatous primary brain tumors show variable FDG uptake. The choline-based tracers seem to be promising in these patients. However, no larger patient series are available. Individually designed protocols for diagnosis and follow-up in patients with these infrequent brain tumors may evolve with the use of coregistered PET and MRI.
In conclusion, CT and particularly MRI, with the administration of intravenous contrast agents for evaluation of the integrity of the blood–brain barrier, are the methods of choice for evaluating nongliomatous brain tumors. FDG-PET and 201Tl SPECT can be used to differentiate recurrent tumor from radiation injury. Coregistration to MRI should be applied whenever possible to characterize the underlying anatomical structures. If suspicious lesions on MRI show FDG uptake at or above that of intact gray matter, tumor recurrence should be suspected.
Introduction
For the diagnosis of malignancies, imaging methods with high contrast between the malignancy and the surrounding tissue are attractive. Classically normal and abnormal brain anatomy is mapped with CT. In the last two decades, MRI has superseded CT in the evaluation of most patients with brain disease, yielding detailed anatomical and also some functional information. The highest degree of functional information is, however, obtained with the use of radiotracers, and here PET imaging and SPECT imaging have a predominant role. Using compounds labeled with positron-emitting or gamma ray–emitting short-lived radioisotopes, it is possible to gain information on flow, metabolism, and receptor systems with tracer doses down to picomolar concentrations in a tissue.
The use of fluorodeoxyglucose PET (FDG-PET) in the diagnosis of intracranial malignancies has been less successful than PET diagnosis and staging of malignancies in other organs. The healthy brain accounts for approximately 20% to 25% of the total body glucose consumed. Thus, the lesion-to-background ratio is particularly unfavorable in this organ when using FDG to delineate a tumor.
The uptake and concentration of a radiolabeled compound in the tissue will depend on several factors. These include blood flow, which determines how much tracer enters the organ, and the rate of transport from the blood to the tissue, which in the brain often will be limited by the impermeability of the blood–brain barrier to most hydrophilic substances. Further, the volume of distribution and possible binding in the tissue (e.g., metabolic trapping of FDG or binding of ligands to specific receptors) are relevant factors. All these variables form a complex mosaic, and it is not always obvious which mechanism is responsible for a high concentration of a radiotracer in a malignancy. An example is the trapping of the natural amino acid [11C]methionine (MET) in a cerebral malignancy. Although the uptake of this tracer might be assumed to represent increased tumor protein synthesis, the correlation between uptake and synthesis remains poor (1). In fact, the MET uptake primarily represents amino acid transport across the blood–brain barrier through an overexpressed L system. Further, a blood–brain barrier rupture, which is characteristic of high-grade tumors and radiation necrosis, will increase the uptake of MET, other amino acids, and amino acids analogs dependent on this transport. For diagnostic purposes, it is important is to have a high contrast between diseased and normal tissue and less important whether this is due to one or another pathophysiological mechanism. However, understanding the basic pathophysiology is of utmost importance for understanding the nature of current diagnostic procedures and for guiding future developments.
For the use of PET and SPECT in the diagnosis of intracranial nongliomatous malignancies and radiation necrosis, the literature is relatively sparse; is dominated by short communications, small patient series, and retrospective investigations; and exhibits the associated limitations and biases.
Primary Central Nervous System Lymphoma (PCNSL)
Primary central nervous system lymphoma (PCNSL) is a rare form of extranodal non-Hodgkin lymphoma (NHL) confined to the brain. The majority of PCNSLs are histologically indistinguishable from NHLs elsewhere in the body, are usually high grade, and show the B phenotype. PCNSL accounts for 1% to 6% of all intracranial tumors (2). A recent dramatic increase in the incidence has been shown in both immunocompetent and immunosuppressed patients and in patients with congenital or acquired immunodeficiencies. The latter include transplant patients and HIV-infected patients, who have a risk of 1% to 5% and 2% to 6%, respectively (3). The absolute risk of PCNSL in immunocompetent patients, however, is still very low. No larger systematic studies are available on the use of PET or SPECT for grading these patients or for assessing their response to treatment. Most immunocompetent patients receive intensive chemotherapy combined with radiotherapy, which increases survival but also usually affects cognitive performance. Overall survival is considered poor, 25% to 42%, compared with the survival rates for other high-grade extranodal lymphomas (4).
In Hodgkin and non-Hodgkin lymphomas outside the CNS, it is well established that FDG-PET has an important role in staging, monitoring of recurrence, and evaluation of treatment effects (5). In PCNSL, however, the use of FDG-PET for these indications remains to be established in larger prospective patient series (6). PCNSLs are highly proliferative tumors and usually show very high levels of FDG uptake (Fig. 23.1). In reading a PET scan, it should be kept in mind that some PCNSLs are very sensitive to dexamethasone treatment, which can lead to a marked reduction in the FDG uptake (7). Due to the lack of specificity of FDG for lymphoma, histology therefore remains mandatory for establishing the diagnosis for PCNSL as well as the diagnosis for extracerebral lymphoma (5,6). As for the gliomatous brain tumors (see Chapter 22), FDG-PET can probably be used to optimize the diagnostic quality of a stereotactic biopsy. Of the other PET tracers, only few have been applied. In a study of 10 subjects with biopsy-proven PCNSL, MET-PET was found useful for lesion delineation and for monitoring the therapeutic effect of irradiation (8).
In HIV-infected or AIDS patients with contrast-enhancing brain tumors on CT or MRI, studies suggest that FDG-PET may help to discriminate CNS lymphoma from inflammatory lesions (e.g., toxoplasmosis) with an accuracy of 80% to 95% (9,10,11). O’Doherty et al. (11) reported that the standardized uptake value (SUV; see Chapter 11) in cerebral lesions of toxoplasmosis was in the range of 0.14 to 3.7 (N = 13) and in cerebral lesions of lymphoma was in the range of 3.9 to 8.7 (N = 6). Three additional patients with progressive multifocal leukoencephalopathy had SUVs in the range of 1.0 to 1.5 in the lesions (11). FDG-PET therefore seems useful for differentiating between lymphomas and infectious lesions in AIDS patients, as also reported in two previously cited studies (9,10).
Thallium 201 (201Tl) SPECT is thought to represent blood flow and blood–brain barrier permeability on early images, whereas delayed images are thought to represent viable tumor tissue. This technique has been used to solve the same differential diagnostic problem, and most studies have reported moderate to high sensitivities (83% to 100%) and specificities (83% to 100%) (12,13,14,15,16,17). However, direct pathological confirmation was performed in fewer than 50% of the cases, and some patients were on steroid treatment. In studies with pathology performed on steroid-naïve patients, the sensitivity (60%) and specificity (55%) were found to be too small to endorse the use of the
method (18), probably reflecting the diverse mechanisms of tracer uptake. The limitations of using 201Tl SPECT reside in the low energy peak, which leads to significant soft-tissue attenuation, unfavorable dosimetry (15 to 20 mSv per scan), and limited spatial resolution.
method (18), probably reflecting the diverse mechanisms of tracer uptake. The limitations of using 201Tl SPECT reside in the low energy peak, which leads to significant soft-tissue attenuation, unfavorable dosimetry (15 to 20 mSv per scan), and limited spatial resolution.
Although the use of FDG-PET and 201Tl SPECT scans seems promising, the effective antiviral therapies for AIDS introduced in recent years have reduced the prominence of the diagnostic problem of determining whether an intracerebral mass is due to infection or lymphoma in AIDS patients. For patients suspected of PCNSL with inconclusive biopsies or cerebrospinal fluid (CSF) cytologies or patients who cannot undergo surgery due to involvement of deep vital structures, a diagnosis based on radiological MR criteria may be acceptable for routine clinical practice. Confirmatory FDG-PET and 201Tl SPECT scans can be recommended, a very high uptake being suggestive of PCNSL, but these investigation are not mandatory (4). It should be noted that FDG-PET cannot differentiate PCNSL from metastasis or glioblastoma multiforme (6).
Nongliomatours Primary Brain Tumors
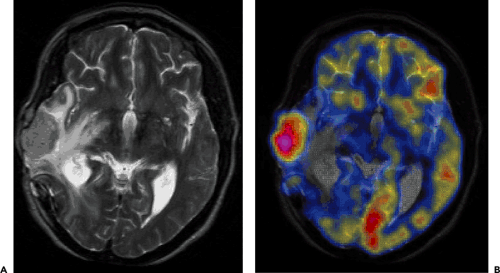
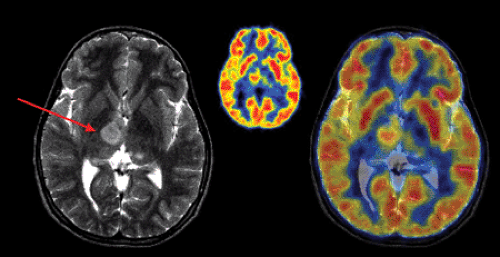
The most common nongliomatous primary brain tumors are meningioma (15% to 20%); tumors of the nerve sheet cell, including neurinoma, schwannoma, and neurofibroma (6% to 8%); and pituitary adenoma (6% to 8%). For these tumors, no larger series document the use of PET for diagnosis, recurrence monitoring, or treatment monitoring. MRI and CT scanning with intravenous contrast enhancement are the methods of choice for diagnosis and follow-up. However, studies of small patient series show that meningioma and schwannoma have increased FDG uptake (Fig. 23.2), and they even indicate that the degree of FDG uptake could be used as a prognostic factor (19,20). About 4% to 5% of patients with neurofibromatosis type 1 (NF-1) develop malignant peripheral nerve sheath tumors, which arise within plexiform neurofibromas and often carry a poor prognosis. In extracranial neurofibromas, FDG-PET allows discrimination of benign neurofibromas from malignant neurogenic peripheral nerve sheath tumors (MPNSTs). FDG-PET also seems to be able to monitor intracranial malignant degeneration of unidentified bright objects (OBSs) on MRI (Fig. 23.3). Pituitary adenomas are generally benign tumors, but they may show marked uptake on FDG (Fig. 23.4) (21).
The use of MET has been investigated in smaller patient series in patients with meningioma. Iuchi et al. (22) found that the proliferative activity of meningioma varied from tumor to tumor and even from tumor region to tumor region in individual patients. They reported that MET uptake correlated significantly with the Ki-67 proliferation index. MET uptake is low in benign lesions and in radiation necrosis. However, high uptake has been described in acute infarctions and acute inflammatory processes (23).
In a recent study by Chung et al. (24) on the use of MET-PET in the evaluation of brain lesions that are hypo- or isometabolic on FDG-PET for patients with meningioma,
these meningiomas had variable but significant MET uptake. MET-PET scans can be brought into stereotactic radiation treatment planning and have been found to improve the target volume definition of meningioma (25).
these meningiomas had variable but significant MET uptake. MET-PET scans can be brought into stereotactic radiation treatment planning and have been found to improve the target volume definition of meningioma (25).
The membrane proliferation tracer [11C]choline was examined in a recent study of 22 patients suspected of having brain tumors (26). One patient with a meningioma, two patients with schwannoma, one patient with craniopharyngioma, and one patient with malignant lymphoma all showed markedly increased [11C]choline uptake in their brain lesions, with images displayed as PET-MRI coregistered images. Areas of [11C]choline accumulation on the PET scans were generally larger than the corresponding areas on the MRI scans (26).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree