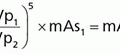Breast Imaging: Mammography
8.0 INTRODUCTION
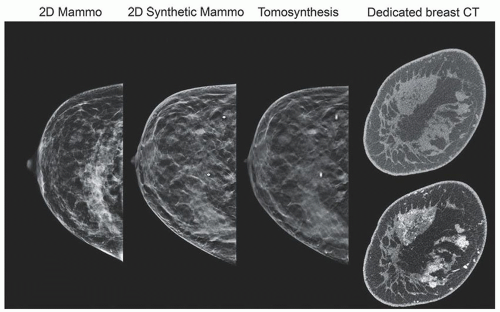
Mammography is a radiographic examination that is optimized for detecting breast cancer. Breast cancer screening with mammography can catch cancers at an earlier, more treatable stage. Technological advances over the last several decades have greatly improved the diagnostic sensitivity of mammography (Fig. 8-1).
Breast cancer screening programs depend on x-ray mammography as a low-cost, low-radiation dose procedure. Mammographic features include masses with irregular or “spiculated” margins, clusters of microcalcifications, and architectural distortions of breast structures.
Screening mammography: detection of breast cancer in asymptomatic population
2 views of each breast, in the mediolateral oblique and craniocaudal views
Diagnostic mammography: assess lesions identified by screening mammography
Includes additional x-ray projections, spot compression and magnification, tomosynthesis, ultrasound, MRI, breast CT, scintigraphy, PET/CT
X-ray attenuation between normal and cancer tissue is small, requiring low energy (Fig. 8-2)
Requirements: high contrast sensitivity, low-dose, high spatial resolution images
Implementation essentials: dedicated x-ray equipment (Fig. 8-3)
8.1 X-RAY TUBE COMPONENTS, STRUCTURES, AND OPERATION
1. Cathode: filament current and tube current are limited. Typical maximum tube current values: 100 to 125 mA for 0.3 mm (large focal spot); 15 to 30 mA for 0.1 mm (small focal spot).
2. Anode: target materials include molybdenum (Mo), rhodium (Rh), and tungsten (W).
a. Anode angle for 65 to 70 cm SID is approximately 22° (with tube tilt) for field coverage of 24 × 30 cm (Fig. 8-4)
b. Half field beam geometry—protects the lungs from radiation—central axis is tangent to the chest wall
c. Heel effect—optimal orientation of x-ray tube: cathode chest wall; anode over anterior (Fig. 8-5)
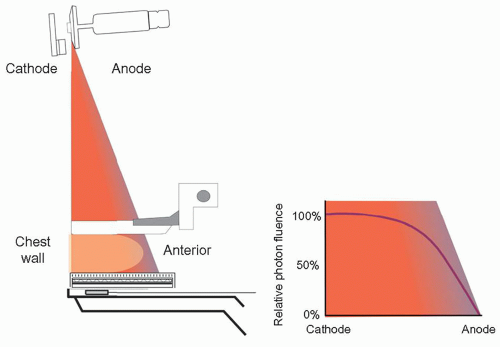
▪ FIGURE 8-5 X-ray tube orientation: cathode over the chest wall and anode over anterior side of the breast to compensate for the heel effect, a loss of x-ray fluence by anode self-filtration.
3. Focal Spot
a. Focal spot—0.3- and 0.1-mm spot size—for contact and magnification studies, respectively
b. Nominal size is measured at a reference axis, halfway between chest wall and anterior dimension of the large field area
c. Best resolution occurs on anterior side of the field per the line focus principle (Fig. 8-6A)
d. System resolution: combination of geometric blurring and detector sampling resolution
(i) Measurement: line-pair bar pattern consisting of frequencies from 4 to 20-lp/mm (Fig. 8-6B)
4. Target, Tube Port. Filtration, Beam Quality: generate x-rays; shape the x-ray photon spectrum.
a. Target; Mo, Rh, W.: Mo and Rh produce useful characteristic x-rays.
b. Tube Port: Beryllium (Z = 4; 0.5 to 1 mm) allows high transmission of all x-ray energies.
c. Tube filtration: Material and thickness remove low- and high-energy x-rays (Fig. 8-7).
d. Characteristic x-rays: Mo: 17 to 19 keV; Rh: 20 to 23 keV; W: 8 to 12 keV—L-x-rays are not useful.
e. Mo target filters used: 30 µm Mo; 25 µm Rh; for thin and thick breasts, respectively (Fig. 8-8)
f. Rh target filters: 25 µm Rh; 25 µm Ag for thicker breasts (Fig. 8-9)
g. W target filters: 50 µm Rh; 50 µm Ag; 500 to 700 µm Al. Thicker filters are required to eliminate unwanted L-x-rays in the spectrum (Fig. 8-10A); Al filter is used to achieve higher output rate with higher energy bremsstrahlung x-rays (Fig. 8-10B)
5. Half Value Layer: The half-value layer (HVL) of a mammography x-ray beam is the thickness in mm of sheets of pure Al required to reduce the x-ray beam air kerma by one-half.
a. HVL depends on kV, target material (Mo, Rh, W), filter material (Mo, Rh, Ag, Al), and filter thicknesses typically used—between 0.3 and 0.7 mm Al (Fig. 8-11).
b. Minimum HVL > kV/100 for mammography (e.g., for 30 kV beam HVL>0.3 mm Al).
c. HVL of breast tissue depends on breast density and kV; typically ranges from 1 to 3 cm tissue thickness.
6. Tube output and output rate: Air kerma in mGy normalized to 100 mAs at a specified distance from the source (focal spot); 50 cm is used in this chapter.
a. Mo and W target output for various filters and thicknesses are shown as a function of kV (Fig. 8-12).
b. Calibrated output values: X mGy (per 100 mAs at 50 cm); entrance surface air kerma, Z mGy, to the breast for Y mAs at a source-to-breast surface distance, D includes inverse square law distance correction:

7. X-ray tube collimation-detector-light field alignment
a. Beam-limiting devices: chest wall edge of x-ray field extends to edge of receptor
(i) X-ray field/receptor congruence: to not exceed 2% of SID
b. X-ray field/light field congruence (Fig. 8-13)—misalignment (length or width) shall not exceed 2% of SID for anterior-posterior or left-right borders.
c. The chest wall edge of compression paddle: shall not extend beyond the chest wall edge of receptor by greater than 1% SID; vertical edge of compression paddle shall not be visible in the image.
d. Small breast imaging: left and right shift collimation accommodates MLO acquisitions to allow for positioning of the arm and shoulder at the corner of the detector (textbook Fig. 8-15).
8.2 X-RAY GENERATOR
1. Automatic Exposure Control (AEC): radiation sensors measure transmitted radiation
a. Densest part of the breast targeted to achieve appropriate SNR
b. AEC circuit assembly is detailed in Figure 8-14.
c. Sensors: for CR, under the detector; for flat-panel, generated by the detector electronics
(i) Manual positioning by the user from posterior to anterior
(ii) Automatic positioning (digital detector) at the highest attenuation in breast
d. Operation: charge accumulates, is amplified, and converted to voltage; compared to reference voltage; when equal, the exposure is terminated
2. AEC algorithms: use compressed breast thickness, kV, tube anode selection (if available), tube filter, and AEC settings on generator control panel as inputs to determine exposure
a. Fully automatic: 100 ms test exposure determines breast penetrability—determines kV, filtration, target (Mo and Rh anodes) to achieve an acceptable exposure time. Most often used in the clinic.
b. Automatic kV: 100 ms test exposure, with preset target and filter
c. Automatic exposure time: with preset target, filter, and kV
3. AEC (SNR) exposure selector: adjustment for increasing or decreasing reference voltage in steps of approximately 15%—“0” is baseline; 1 step increments of 1 step from, for example, -3 to +4 (vendor dependent)
4. Overexposure and backup timer—turns off x-ray exposure after predetermined time; protects from sensor failure, low kV, highly attenuating object in FOV—safety switch to protect patient
5. Underexposure situations
a. Thin and fatty-replaced breasts—possible overexposure because of AEC sensor lag
b. Active sensor measures open-field area—results in noisy images (see Fig. 8-16B in text book)
6. Technique charts: guides to determine kV, mAs, and target-filter (see Table 8-2 in text book)
8.3 COMPRESSION, SCATTERED RADIATION, AND MAGNIFICATION
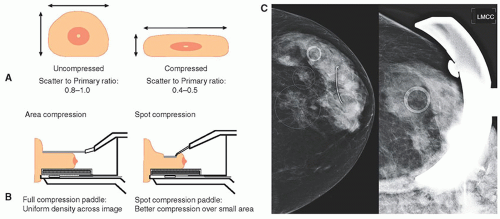
1. Breast compression: reduces overlapping anatomy, decreases tissue thickness for less geometric blurring, stops inadvertent motion, lowerradiation dose (Fig. 8-15A).
a. Compression paddle—Lexan plate attached to a mechanical assembly (Fig. 8-15B)
(i) Right-angle edge at the chest wall produces a flat, uniform breast thickness
(ii) Alternative: “flex” paddle is spring-loaded on the anterior side; curved paddle.
b. Spot compression: approximately 7-cm diameter region, for aggressive compression (Fig. 8-15C)
c. Compression force (optimal)—111 to 200 newtons (25 to 44 lb)
d. Foot pedal motor-driven adjustment is accompanied with a mechanical adjustment knob
2. Scattered radiation: additive, varying distribution degrades subject contrast, adds quantum noise
a. Scatter to primary ratio (SPR) is a function of breast thickness and field area (Fig. 8-16)
b. Contrast degradation factor—amount of contrast lost by detection of scatter—subject contrast without scatter—C0; contrast with of scatter, CS

3. Antiscatter grids
a. Transmission: 60% to 70% of primary x-rays; absorption of 75% to 85% of scattered x-rays
b. Linear focused grids: ratios of 4:1 to 5:1, frequencies of 30 to 45/cm (Fig. 8-17A)
c. Cellular grids: copper honeycomb with air interspace, approximately 3.8:1 ratio, achieves 2D scatter compensation (Fig. 8-17B)
d. Grid motion during the exposure blurs the grid structure
e. Short exposures cause most gridline artifacts due to insufficient motion.
f. Increased dose for using grids is about a factor of approximately 2×.
4. Air gap: large fraction of scatter misses the detector.
a. Achieved with magnification (Fig. 8-17C)
b. Anatomic field of view (FOV) is reduced because of magnification.
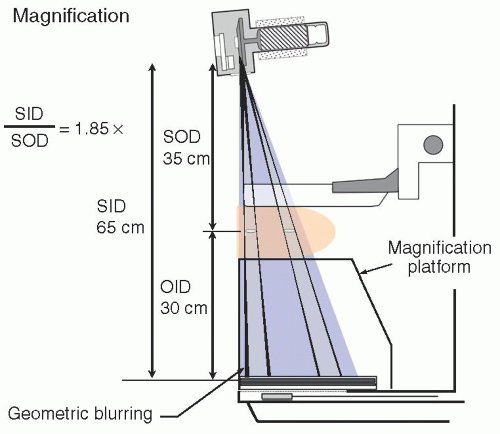
5. Magnification: improves overall mammography system resolution
a. Breast support platform (magnification stand) positions breast well above detector.
b. Small (0.1 mm) focal spot is required to reduce geometric blurring.
c. No antiscatter grid, compression paddles designed for magnification (Fig. 8-18)
d. Typical geometric magnifications: 1.5×, 1.8×, or 2.0×
e. Advantages: Increased spatial resolution, reduction of quantum noise and scatter
f. Limitations: geometric blurring, tube current limit: approximately 25 mA; exposure time: approximately 4 s
g. Breast dose is similar to contact imaging—this results from the offset of no grid ↓(reduced dose) versus inverse square distance ↑(increased dose).
8.4 DIGITAL ACQUISITION SYSTEMS
1. Full-field digital mammography (FFDM)
a. Technology: flat panel thin film transistor (TFT) array detector (Fig. 8-19)
b. Detection: indirect and direct x-ray detection (Fig. 8-20)
(i) Indirect—conversion of x-rays to light to charge
(ii) Direct—conversion of x-rays directly to charge
c. CMOS (Complementary Metal Oxide Semiconductor)—crystalline silicon approximately 50-75 µm dexels, uses an indirect mode of x-ray signal acquisition; functionally similar to TFT arrays
d. Computed Radiography (CR)—cassette-based transition technology for digital mammo (no longer used)
e. Benefits (over screen-film mammography)
(i) Wide dynamic range with separation of acquisition and display
(ii) “For Processing” (raw, corrected) images for quantitation, CAD, AI evaluation
(iii) “For Presentation” enhance contrast, skin-line visibility, magnification, edges.
(iv) Lower dose for flat-panel TFT and CMOS detectors; MGD for 4.2 cm breast—approximately 1 to 1.5 mGy relative to 1.8 to 2.2 mGy for screen/film and CR (see dosimetry section)
f. Increased productivity (but not CR): no handling of cassettes, instant image processing
2. Digital tomosynthesis—a method to reduce the problem of anatomical superimposition
a. Projection images acquired over a range of angles generate depth-dependent shifts.
(i) Reconstruction: “shift and add” or filtered backprojection methods (Fig. 8-21)
b. The x-ray tube/detector system acquires projection images over a limited angle arc.
(i) Tomographic angles from ±7.5° (15°) to ±25° (50°)—larger tomographic angles provide better Z-axis resolution (Fig. 8-22).
(ii) Acquisition times from approximately 5 s up to approximately 25 s dependent on tomographic angle range in continuous or “step and shoot” mode
(iii) Many vendors and systems use detector binning mode (2 × 2) dexel output.
(iv) DBT system design parameters are shown in Table 8-3 (in the textbook).
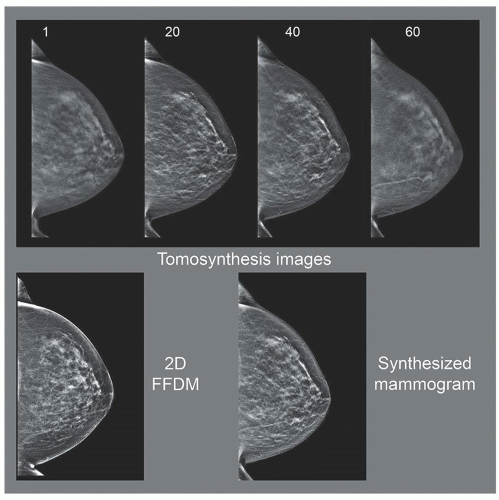
c. Tomosynthesis images are reconstructed approximately every 1 mm plus 2 to 3 images beyond the breast skin on each side; for example, 50 mm compressed breast thickness generates about 55 images in Z plane
d. Image viewing: stacked mode, parallel to detector plane, with 1 mm spacing (Fig. 8-23)
e. Image quality: number of projections affects in-plane resolution and blurring artifacts.
f. Synthetic mammography—realign and create a projection image, like conventional 2D.
(i) Eliminates need for a conventional screening mammogram; reduces MGD by 50%.
(ii) Reduced resolution, some calcification (over) enhancement
g. MGD: approximately 10% to 15% higher than 2D; antiscatter grid is used for some systems.
h. Artifacts: incomplete blurring/suppression of high-density objects, with “halo” signs; anatomy close to focal plane appears larger; truncation artifacts at the reconstructed image periphery; stairstep artifacts with objects unintentionally placed in the beam, such as the shoulder
3. Dedicated breast CT—true 3D volume datasets and elimination of superimposition
a. Cone beam CT geometry in horizontal plane (Fig. 8-24) using a flat panel detector
b. Hundreds of low-dose images acquired about the breast position without compression
c. Reconstruction of the 2D images into nonoverlapping voxels in the breast volume
d. Higher kV (˜60 kV) permits lower radiation dose; a scatter grid is not typically used.
e. MGD: like a two-view mammogram—for example, a 4.5 cm compressed breast ×2 = approximately 4 mGy
f. Breast CT with contrast is thought to be largely equivalent to breast MRI.
4. Stereotactic biopsy—systems localize and sample breast lesions in 3D
a. Use: biopsy of microcalcification lesions (breast mass biopsy—ultrasound or MRI)
b. Geometry configurations
(i) Prone table—breast placed through hole in horizontal table (Fig. 8-25)
(ii) Standard mammography system—add-on biopsy device for lesion trajectory/depth
c. Two images acquired ±15° (30° total) from the conventional projection (Fig. 8-26)
(i) Objects closer to the detector shift less than objects further away (toward the focal spot)
d. Depth and trajectory: determined by the location and shift in each image (Fig. 8-26)
 ▪ FIGURE 8-25 A stereotactic biopsy system with a prone positioning system is shown. The breast is positioned through the hole onto the detector platform and compressed by an open-access paddle (close-up shown on the right, with the x,y,z coordinate directions indicated). The x-ray tube pivots horizontally about a fulcrum point to acquire digital images at +15° and -15° projections.
 F. 8-28 F. 8-28Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|