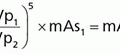1. Ionization is the removal of electrons from atoms or molecules. (An atom or molecule stripped of an electron has a net positive charge and is called a cation. In many gases, the free electrons become attached to uncharged atoms or molecules, forming negatively charged anions. An ion pair consists of a cation and its associated free electron or anion.)
2. Excitation is the elevation of electrons to excited states in atoms, molecules, or a crystal. Excitation and ionization may produce chemical changes or the emission of visible light or ultraviolet (UV) radiation. Most energy deposited by ionizing radiation is ultimately converted into thermal energy.
1. A gas-filled detector consists of a volume of gas between two electrodes. Ions produced in the gas by the radiation are collected by the electrodes, resulting in an electrical signal.
2. The interaction of ionizing radiation with certain materials produces UV radiation and/or visible light. These materials are called scintillators. They are commonly attached to or incorporated in devices that convert UV radiation and light into an electrical signal.
3. Semiconductor detectors are especially pure crystals of silicon, germanium, or other semiconductor materials to which trace amounts of impurity atoms have been added so that they act as diodes. A diode is an electronic device with two terminals that permits a large electrical current to flow when a voltage is applied in one direction, but very little current when the voltage is applied in the opposite direction.
1. Detectors, such as Geiger-Müeller (GM) detectors, that indicate the number of interactions occurring in the detector are called counters.
2. Detectors that yield information about the energy distribution of the incident radiation, such as NaI scintillation detectors, are called spectrometers.
3. Detectors that indicate the net amount of energy deposited in the detector by multiple interactions are called dosimeters.
1. Many radiation detectors produce an electrical signal after each interaction of a particle or photon. The signal generated by the detector passes through a series of electronic circuits, each of which performs a function such as signal amplification, signal processing, or data storage.
2. A detector and its associated electronic circuitry form a detection system. There are two fundamental ways that the circuitry may process the signal—pulse mode and current mode. In pulse mode, the signal from each interaction is processed individually. In current mode, the electrical signals from individual interactions are averaged together, forming a net current signal.
3. There are advantages and disadvantages to each method of handling the signal. GM detectors are operated in pulse mode, whereas most ionization chambers, including ion chamber survey meters and the dose calibrators used in nuclear medicine, are operated in current mode.
4. Scintillation detectors are operated in pulse mode in nuclear medicine applications, but in current mode in direct digital radiography, fluoroscopy, and x-ray computed tomography (CT).
5. In this chapter, the term interaction typically refers to the interaction of a single photon or charged particle, such as the interaction of a gamma-ray by the photoelectric effect or Compton scattering.
6. The term event may refer to a single interaction, or it may refer to something more complex, such as two nearly simultaneous interactions in a detector. In instruments that process the signals from individual interactions or events in pulse mode, an interaction or event that is registered is referred to as a count.
1. The main problem with using a radiation detector or detection system in pulse mode is that two interactions must be separated by a finite amount of time if they are to produce distinct signals. This interval is called the dead time of the system.
2. If a second interaction occurs during this time interval, its signal will be lost; furthermore, if it is close enough in time to the first interaction, it may even distort the signal from the first interaction. The fraction of counts lost from dead-time effects is smallest at low interaction rates and increases with increasing interaction rate.
3. The dead time of a detection system is largely determined by the component in the series with the longest dead time. For example, the detector usually has the longest dead time in GM counter systems, whereas in multichannel analyzer (MCA) systems (see later discussion), the analog-to-digital converter (ADC) generally has the longest dead time.
4. The dead times of different types of systems vary widely. GM counters have dead times ranging from tens to hundreds of microseconds, whereas most other systems have dead times of less than a few microseconds. It is important to know the count-rate behavior of a detection system; if a detection system is operated at too high an interaction rate, an artificially low count rate will be obtained.
5. There are two mathematical models describing the behavior of detector systems operated in pulse mode—paralyzable and nonparalyzable. Although these models are simplifications of the behavior of real detection systems, real systems may behave like one or the other model. In a paralyzable system, an interaction that occurs during the dead time after a previous interaction extends the dead time; in a nonparalyzable system, it does not.
6. Figure 17-1 shows the count rates of paralyzable and nonparalyzable detector systems as a function of the interaction rate in the detector.
1. When a detector is operated in current mode, all information regarding individual interactions is lost. For example, neither the interaction rate nor the energies deposited by individual interactions can be determined.
2. However, if the amount of electrical charge collected from each interaction is proportional to the energy deposited by that interaction, then the net electrical current is proportional to the dose rate in the detector material.
3. Detectors subject to very high interaction rates are often operated in current mode to avoid dead-time information losses. Image-intensifier tubes and flat-panel image receptors in fluoroscopy, detectors in x-ray CT machines, direct digital radiographic image receptors, ion chambers used in phototimed radiography, and most nuclear medicine dose calibrators are operated in current mode.
1. The term spectroscopy, literally the viewing of a spectrum, is commonly used to refer to measurements of the energy distributions of radiation fields, and a spectrometer is a detection system that yields information about the energy distribution of the incident radiation.
2. Most spectrometers are operated in pulse mode, and the amplitude of each pulse is proportional to the energy deposited in the detector by the interaction causing that pulse.
3. The energy deposited by an interaction, however, is not always the total energy of the incident particle or photon. For example, a gamma-ray may interact with the detector by Compton scattering, with the scattered photon escaping the detector. In this case, the deposited energy is the difference between the energies of the incident and scattered photons.
4. A pulse height spectrum is usually depicted as a graph of the number of interactions depositing a particular amount of energy in the spectrometer as a function of energy (Fig. 17-2). Because the energy deposited by an interaction may be less than the total energy of the incident particle or photon and also because of random variations in the detection process, the pulse height spectrum produced by a spectrometer is not identical to the actual energy spectrum of the incident radiation.
5. The energy resolution of a spectrometer is a measure of its ability to differentiate between particles or photons of different energies. Pulse height spectroscopy is discussed later in this chapter.
1. The efficiency (or sensitivity) of a detector is a measure of its ability to detect radiation. The efficiency of a detection system operated in pulse mode is defined as the probability that a particle or photon emitted by a source will be detected.
2. It is measured by placing a source of radiation in the vicinity of the detector and dividing the number of particles or photons detected by the number emitted:

This equation can be written as follows:

3. Therefore, the detection efficiency is the product of two terms: the geometric efficiency and the intrinsic efficiency:

where the geometric efficiency of a detector is the fraction of emitted particles or photons that reach the detector and the intrinsic efficiency is the fraction of those particles or photons reaching the detector that are detected. Because total, geometric, and intrinsic efficiencies are all probabilities, each ranges from 0 to 1.
4. The geometric efficiency is determined by the geometric relationship between the source and the detector (Fig. 17-3). It increases as the source is moved toward the detector and approaches 0.5 when a point source is placed against a flat surface of the detector because in that position-half of the photons or particles are emitted into the detector.
5. For a source inside a well-type detector, the geometric efficiency approaches 1, because most of the particles or photons are intercepted by the detector. A well-type detector is a detector containing a cavity for the insertion of samples.
6. The intrinsic efficiency of a detector in detecting photons also called the quantum detection efficiency (QDE) is determined by the energy of the photons and the atomic number, density, and thickness of the detector.
7. If a parallel beam of monoenergetic photons is incident upon a detector of uniform thickness, the intrinsic efficiency of the detector is given by the following equation:

where µ is the linear attenuation coefficient of the detector material, ρ is the density of the material, µ/ρ is the mass attenuation coefficient of the material, and x is the thickness of the detector.
8. This equation shows that the intrinsic efficiency for detecting x-rays and gamma rays increases with the thickness of the detector and the density and the mass attenuation coefficient of the detector material. The mass attenuation coefficient increases with the atomic number of the material and, within the range of photon energies used in diagnostic imaging, decreases with increasing photon energy, with the exception of absorption edges.
1. As shown in Figure 17-4, a gas-filled detector consists of a volume of gas between two electrodes, with an electric potential difference (voltage) applied between the electrodes.
2. Ionizing radiation forms ion pairs in the gas. The positive ions (cations) are attracted to the negative electrode (cathode), and the electrons or anions are attracted to the positive electrode (anode). In most detectors, the cathode is the wall of the container that holds the gas or a conductive coating on the inside of the wall, and the anode is a wire inside the container. After reaching the anode, the electrons travel through the circuit to the cathode, where they recombine with the cations. This electrical current can be measured with a sensitive ammeter or other electrical circuitry.
3. There are two types of gas-filled detectors in common use—ionization chambers and GM counters. The type of detector is determined primarily by the voltage applied between the two electrodes.
a. In an ionization chamber, the two electrodes can have almost any configuration: they may be two parallel plates, two concentric cylinders, or a wire within a cylinder.
b. In GM counters, the anode must be a thin wire.
4. Figure 17-5 shows the amount of electrical charge collected after a single interaction as a function of the electrical potential difference (voltage) applied between the two electrodes.
5. Ionizing radiation produces ion pairs in the gas of the detector. If no voltage is applied between the electrodes, no current flows through the circuit because there is no electric field to attract the charged particles to the electrodes; the ion pairs merely recombine in the gas. When a small voltage is applied, some of the cations are attracted to the cathode and some of the electrons or anions are attracted to the anode before they can recombine. As the voltage is increased, more ions are collected, and fewer recombine. This region, in which the current increases as the voltage is raised, is called the recombination region of the curve.
6. As the voltage is increased further, a plateau is reached in the curve. In this region, called the ionization chamber region, the applied electric field is sufficiently strong to collect almost all ion pairs; additional increases in the applied voltage do not significantly increase the current.
7. Beyond the ionization region, the collected current again increases as the applied voltage is raised. In this region, called the proportional region, electrons approaching the anode are accelerated to such high kinetic energies that they cause additional ionization. This phenomenon, called gas multiplication, amplifies the collected current; the amount of amplification increases as the applied voltage is raised. At any voltage through the ionization chamber region and the proportional region, the amount of electrical charge collected from each interaction is proportional to the amount of energy deposited in the gas of the detector by the interaction. For example, the amount of charge collected after an interaction depositing 100 keV is one tenth of that collected from an interaction depositing 1 MeV.
8. Beyond the proportional region is a region in which the amount of charge collected from each event is the same, regardless of the amount of energy deposited by the interaction. In this region, called the Geiger-Müeller region (GM region), the gas multiplication spreads the entire length of the anode. The size of a pulse in the GM region tells nothing about the energy deposited in the detector by the interaction causing the pulse.
1. Because gas multiplication does not occur at the relatively low voltages applied to ionization chambers, the amount of electrical charge collected from a single interaction is very small and would require huge amplification to be detected. For this reason, ionization chambers are seldom used in pulse mode. The advantage to operating them in current mode is the almost complete freedom from dead-time effects, even in very intense radiation fields.
2. Almost any gas can be used to fill the chamber. If the gas is air and the walls of the chamber are of a material whose effective atomic number is similar to air, the amount of current produced is proportional to the exposure rate (exposure is the amount of electrical charge produced per mass of air).
3. Air-filled ion chambers are used in portable survey meters and can accurately indicate exposure rates from less than 1 mR/h to tens or hundreds of roentgens per hour. Air-filled ion chambers are also used for performing quality assurance testing of diagnostic and therapeutic x-ray machines, and they are the detectors in most x-ray machine phototimers.
4. Measurements using an air-filled ion chamber that is open to the atmosphere are affected by the density of the air in the chamber, which is determined by ambient air pressure and temperature. Measurements using such chambers that require great accuracy must be corrected for these factors.
5. Gas-filled detectors tend to have low intrinsic efficiencies for detecting x-rays and gamma rays because of the low densities of gases and the low atomic numbers of most common gases. The sensitivity of ion chambers to x-rays and gamma rays can be enhanced by filling them with a gas that has a high atomic number, such as argon (Z = 18) or xenon (Z = 54), and pressurizing the gas to increase its density. Well-type ion chambers called dose calibrators are used in nuclear medicine to assay the activities of dosages of radiopharmaceuticals to be administered to patients; many are filled with pressurized argon. Xenon-filled pressurized ion chambers were formerly used as detectors in some CT machines.
6. Air-filled ion chambers are commonly used to measure the related quantities air kerma and exposure rate. Air kerma is the initial kinetic energy transferred to charged particles (in this case electrons), liberated in air by the radiation, per mass air, while exposure is the amount of electrical charge created by these electrons in air caused by ionization, per mass air.
7. There is a problem measuring the ionization in the small volume of air in an ionization chamber of reasonable size. The energetic electrons released by interactions in the air have long ranges in air, and many of them would escape the air in the chamber and cause much of their ionization elsewhere.
8. This problem can be partially solved by building the ion chamber with thick walls of a material whose effective atomic number is similar to that of air. In this case, the number of electrons escaping the volume of air is approximately matched by a similar number of electrons released in the chamber wall entering the air in the ion chamber. This situation, if achieved, is called electronic equilibrium.
9. For this reason, most ion chambers for measuring exposure or air kerma have thick air equivalent walls or are equipped with removable air equivalent buildup caps to establish electronic equilibrium. The thickness of material needed to establish electronic equilibrium increases with the energy of the x- or gamma rays.
10. However, thick walls or buildup caps may significantly attenuate low energy x- and gamma rays. Many ion chamber survey meters have windows that may be opened in the thick material around the ion chamber to permit more accurate measurement of low energy x- and gamma rays.
1. GM counters must also contain gases with specific properties. Because gas multiplication produces billions of ion pairs after an interaction, the signal from a GM detector requires little additional amplification. For this reason, GM detectors are often used for inexpensive survey meters.
2. GM detectors have high efficiencies for detecting charged particles that penetrate the walls of the detectors; almost every such particle reaching the interior of a detector is counted. Many GM detectors are equipped with thin windows to allow beta particles and conversion electrons to reach the gas and be detected.
3. Very weak charged particles, such as the beta particles emitted by tritium (3H, Ernax = 18 keV), which is extensively used in biomedical research, cannot penetrate the windows; therefore, contamination by 3H cannot be detected with a GM survey meter. Flat, thin-window GM detectors, called “pancake”-type detectors, are very useful for finding radioactive contamination.
4. The size of the voltage pulse from a GM tube is independent of the energy deposited in the detector by the interaction causing the pulse: an interaction that deposits 1 keV causes a voltage pulse of the same size as one caused by an interaction that deposits 1 MeV. Therefore, GM detectors cannot be used as spectrometers or precise dose-rate meters.
5. Many portable GM survey meters display measurements in units of milliroentgens per hour. However, the GM counter cannot truly measure exposure rates, and so its reading must be considered only an approximation.
6. If a GM survey meter is calibrated to indicate exposure rate for 662-keV gamma rays from 137Cs (commonly used for calibrations), it may overrespond by as much as a factor of 5 for photons of lower energies, such as 80 keV. If an accurate measurement of exposure rate is required, an air-filled ionization chamber survey meter should be used.
7. GM detectors suffer from extremely long dead times, ranging from tens to hundreds of microseconds. For this reason, GM counters are seldom used when accurate measurements are required of count rates greater than a few hundred counts per second. A portable GM survey meter may become paralyzed in a very high radiation field and yield a reading of zero. Ionization chamber instruments should always be used to measure highintensity x-ray and gamma-ray fields.
1. Scintillators are materials that emit visible light or UV radiation after the interaction of ionizing radiation with the material. Scintillators are used in conventional film-screen radiography, many direct digital radiographic image receptors, fluoroscopy, scintillation cameras, CT scanners, and positron emission tomography (PET) scanners.
2. Although the light emitted from a single interaction can be seen if the viewer’s eyes are dark-adapted, most scintillation detectors incorporate a means of signal amplification. In conventional film-screen radiography, photographic film is used to amplify and record the signal. In other applications, electronic devices such as photomultiplier tubes (PMTs), photodiodes, or image-intensifier tubes convert the light into electrical signals. PMTs and image-intensifier tubes amplify the signal as well.
3. A scintillation detector consists of a scintillator and a device, such as a PMT, that converts the light into an electrical signal.
4. When ionizing radiation interacts with a scintillator, electrons are raised to an excited energy level. Ultimately, these electrons fall back to a lower energy state, with the emission of visible light or UV radiation.
5. Most scintillators have more than one mode for the emission of visible light or UV radiation, and each mode has its characteristic decay constant. Luminescence is the emission of light after excitation. Fluorescence is the prompt emission of light, whereas phosphorescence (also called afterglow) is the delayed emission of light.
6. When scintillation detectors are operated in current mode, the prompt signal from an interaction cannot be separated from the phosphorescence caused by previous interactions. When a scintillation detector is operated in pulse mode, afterglow is less important because electronic circuits can separate the rapidly rising and falling components of the prompt signal from the slowly decaying delayed signal resulting from previous interactions. Properties that are desirable in a scintillator:
a. The conversion efficiency, the fraction of deposited energy that is converted into light or UV radiation, should be high. (Conversion efficiency should not be confused with detection efficiency).
b. For many applications, the decay times of excited states should be short. (Light or UV radiation is emitted promptly after an interaction.)
c. The material should be transparent to its own emissions. (Most emitted light or UV radiation escapes reabsorption.)
d. The frequency spectrum (color) of emitted light or UV radiation should match the spectral sensitivity of the light receptor (PMT, photodiode, or film).
e. If used for x-ray and gamma-ray detection, the attenuation coefficient (µ) should be large, so that detectors made of the scintillator have high detection efficiencies. Materials with large atomic numbers and high densities have large attenuation coefficients.
f. The material should be rugged, unaffected by moisture, and inexpensive to manufacture.
7. In all scintillators, the amount of light emitted after an interaction increases with the energy deposited by the interaction. Therefore, scintillators may be operated in pulse mode as spectrometers. When a scintillator is used for spectroscopy, its energy resolution (ability to distinguish between interactions depositing different energies) is primarily determined by its conversion efficiency.
8. There are several categories of materials that scintillate. Many organic compounds exhibit scintillation. In these materials, scintillation is a property of the molecular structure. Solid organic scintillators are used for timing experiments in particle physics because of their extremely prompt light emission. Organic scintillators include the liquid scintillation fluids that are used extensively in biomedical research. Organic scintillators are not used for medical imaging because the low atomic numbers of their constituent elements and their low densities make them poor x-ray and gamma-ray detectors. When photons in the diagnostic energy range do interact with organic scintillators, it is primarily by Compton scattering.
9. Many inorganic crystalline materials exhibit scintillation. In these materials, the scintillation is a property of the crystalline structure: if the crystal is dissolved, the scintillation ceases. Many of these materials have much larger average atomic numbers and higher densities than organic scintillators and therefore are excellent photon detectors. They are widely used for radiation measurements and imaging in radiology.
10. Most inorganic scintillation crystals are deliberately grown with trace amounts of impurity elements called activators. The atoms of these activators form preferred sites in the crystals for the excited electrons to return to the ground state. The activators modify the frequency (color) of the emitted light, the promptness of the light emission, and the proportion of the emitted light that escapes reabsorption in the crystal.
1. No one scintillation material is best for all applications in radiology. Sodium iodide activated with thallium [NaI(Tl)] is used for most nuclear medicine applications. It is coupled to PMTs and operated in pulse mode in scintillation cameras, thyroid probes, and gamma well counters.
2. NaI(Tl) high content of iodine (Z = 53) and high density provides a high photoelectric absorption probability for x-rays and gamma rays emitted by common nuclear medicine radiopharmaceuticals (70 to 365 keV).
3. NaI(Tl) has a very high conversion efficiency; approximately 13% of deposited energy is converted into light. Because a light photon has an energy of about 3 eV, approximately one light photon is emitted for every 23 eV absorbed by the crystal. This high conversion efficiency gives it a very good energy resolution.
4. NaI(Tl) emits light very promptly (decay constant, 250 ns), permitting it to be used in pulse mode at interaction rates greater than 100,000/s. Very large crystals can be manufactured; for example, the rectangular crystals of one modern scintillation camera are 59 cm (23 inches) long, 44.5 cm (17.5 inches) wide, and 0.95 cm thick. Unfortunately, NaI(Tl) crystals are fragile; they crack easily if struck or subjected to rapid temperature change. Also, they are hygroscopic (i.e., they absorb water from the atmosphere) and therefore must be hermetically sealed.
5. Positron emission tomography (PET), discussed in Chapter 19, requires high detection efficiency for 511-keV annihilation photons and a prompt signal from each interaction because the signals must be processed in pulse mode at high interaction rates. PET detectors are thick crystals of high-density, high atomic number scintillators optically coupled to PMTs.
6. For many years, bismuth germanate (Bi4Ge3O12, often abbreviated as “BGO”) was the preferred scintillator. The high atomic number of bismuth (Z = 83) and the high density of the crystal yield a high intrinsic efficiency for the 511-keV positron annihilation photons. The primary component of the light emission is sufficiently prompt (decay constant, 300 ns) for PET. NaI(Tl) was used in early and some less-expensive PET scanners.
7. Today, lutetium oxyorthosilicate (Lu2SiO4O, abbreviated LSO), lutetium-yttrium oxyorthosilicate (LuxYSiO4O, abbreviated LYSO), and gadolinium oxyorthosilicate (Gd2SiO4O, abbreviated GSO), all activated with cerium, are used in newer PET scanners. Their densities and effective atomic numbers are similar to those of BGO, but their conversion efficiencies are much larger, and they emit light much more promptly.
8. Calcium tungstate (CaWO4) is used in CT scanners. It was also used for many years in intensifying screens in film-screen radiography. It was largely replaced by rare earth phosphors, such as gadolinium oxysulfide activated with terbium. The intensifying screen is an application of scintillators that does not require very prompt light emission because the film usually remains in contact with the screen for at least several seconds after exposure.
9. Cesium iodide activated with thallium is used as the phosphor layer of many indirect-detection thin-film transistor radiographic and fluoroscopic image receptors, described in Chapters 7 and 9. Cesium iodide activated with sodium is used as the input phosphor, and zinc cadmium sulfide activated with silver is used as the output phosphor of image-intensifier tubes in fluoroscopes.
TABLE 17-1 INORGANIC SCINTILLATORS USED IN MEDICAL IMAGING | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. PMTs perform two functions—conversion of UV and visible light photons into an electrical signal and signal amplification, on the order of millions to billions.
2. As shown in Figure 17-6, a PMT consists of an evacuated glass tube containing a photocathode, typically 10 to 12 electrodes called dynodes, and an anode. The photocathode is a very thin electrode, located just inside the
glass entrance window of the PMT, which emits electrons when struck by visible light. Photocathodes are inefficient; approximately one electron is emitted from the photocathode for every five UV or light photons incident upon it.
3. A high-voltage power supply provides a voltage of approximately 1,000 V, and a series of resistors divide the voltage into equal increments. The first dynode is given a voltage of about +100 V with respect to the photocathode; successive dynodes have voltages that increase by approximately 100 V per dynode. The electrons emitted by the photocathode are attracted to the first dynode and are accelerated to kinetic energies equal to the potential difference between the photocathode and the first dynode. (If the potential difference is 100 V, the kinetic energy of each electron is 100 eV.)
4. When these electrons strike the first dynode, about five electrons are ejected from the dynode for each electron hitting it. These electrons are then attracted to the second dynode, reaching kinetic energies equal to the potential difference between the first and second dynodes, and causing about five electrons to be ejected from the second dynode for each electron hitting it.
5. This process continues down the chain of dynodes, with the number of electrons being multiplied by a factor of 5 at each stage. The total amplification of the PMT is the product of the individual multiplications at each dynode.
1. Photodiodes are semiconductor diodes that convert light into electrical signals. In use, photodiodes are reverse biased. Reverse bias means that the voltage is applied with the polarity such that essentially no electrical current flows.
2. When the photodiode is exposed to light, an electrical current is generated that is proportional to the intensity of the light. Photodiodes are sometimes used with scintillators instead of PMTs.
3. Photodiodes produce more electrical noise than PMTs do, but they are smaller and less expensive. Most photodiodes, unlike PMTs, do not amplify the signal. However, a type of photodiode called an avalanche photodiode does provide signal amplification, although not as much as a PMT.
4. Photodiodes coupled to CdWO4 or other scintillators are used in current mode in CT scanners. Photodiodes are also essential components of indirect-detection thin-film transistor radiographic and fluoroscopic image receptors, which use scintillators to convert x-ray energy into light.
1. Another solid-state electronic device that can detect and measure visible light and/or UV radiation from a scintillator is a rectangular array of tiny (typically 10 to 100 µm) avalanche photodiodes operated in Geiger mode, with the outputs summed.
2. Such diode arrays have been given several names, including multi-pixel photon counters, but perhaps are most commonly referred to as silicon photomultipliers (SiPMs).
1. Scintillators with electron trapping can be used for dosimetry. In the case of thermoluminescent dosimeters (TLDs), to read the signal after exposure to ionizing radiation, a sample of TLD material is heated, the light is detected and converted into an electrical signal by a PMT, and the resultant signal is integrated and displayed.
2. The amount of light emitted by the TLD increases with the amount of energy absorbed by the TLD but may deviate from proportionality, particularly at higher doses. After the TLD has been read, it may be baked in an oven to release the remaining trapped electrons and reused.
3. Lithium fluoride (LiF) is one of the most useful TLD materials. It is commercially available in forms with different trace impurities (Mg and Ti or Mg, Cu, and P), giving differences in properties such as sensitivity and linearity of response with dose.
4. LiF has trapping centers that exhibit almost negligible release of trapped electrons at room temperature, so there is little loss of information with time from exposure to the reading of the TLD. The effective atomic number of LiF is close to that of tissue, so the amount of light emission is almost proportional to the tissue dose over a wide range of x-ray and gamma-ray energies. It is commonly used instead of photographic film for personnel dosimetry.
1. In optically stimulated luminescence (OSL), the trapped excited electrons are released by exposure to light, commonly produced by a laser, of a frequency optimal for releasing the trapped electrons. The most commonly used OSL material is aluminum oxide (Al2O3) activated with a small amount of carbon.
2. The effective atomic number of aluminum oxide is significantly higher than that of soft tissue, and so the dose to this material is not proportional to the dose to soft tissue over the full range of energies used in medical imaging.
1. Photostimulable phosphors (PSPs), like TLDs, are scintillators in which a fraction of the excited electrons become trapped. PSP plates are used in radiography as image receptors, instead of film-screen cassettes.
2. Although the trapped electrons could be released by heating, a laser is used to scan the plate and release them. The electrons then fall to the ground state, with the emission of light.
3. Barium fluorohalide activated with europium is commonly used for PSP imaging plates. In this material, the wavelength that is most efficient in stimulating luminescence is in the red portion of the spectrum, whereas the stimulated luminescence itself is in the blue-violet portion of the spectrum.
4. The stimulated emissions are converted into an electrical signal by PMTs. After the plate is read by the laser, it may be exposed to light to release the remaining trapped electrons that can be reused.
1. Semiconductors are crystalline materials whose electrical conductivities are less than those of metals but more than those of crystalline insulators. Silicon and germanium are common semiconductor materials.
2. In crystalline materials, electrons exist in energy bands, separated by forbidden gaps. In metals (e.g., copper), the least tightly bound electrons exist in a partially occupied band, called the conduction band. The conductionband electrons are mobile, providing high electrical conductivity.
3. In an insulator or a semiconductor, the valence electrons exist in a filled valence band. In semiconductors, these valence-band electrons participate in covalent bonds and so are immobile. The next higher energy band, the conduction band, is empty of electrons.
4. However, if an electron is placed in the conduction band, it is mobile, as are the upper band electrons in metals. The difference between insulators and semiconductors is the magnitude of the energy gap between the valence and conduction bands. In insulators, the band gap is greater than 5 eV, whereas in semiconductors, it is about 1 eV or less (Fig. 17-7).
5. In semiconductors, valence-band electrons can be raised to the conduction band by ionizing radiation, visible light or UV radiation, or thermal energy.
6. When a valence-band electron is raised to the conduction band, it leaves behind a vacancy in the valence band. This vacancy is called a hole. Because a hole is the absence of an electron, it is considered to have a net positive charge, equal but opposite to that of an electron.
7. When another valence-band electron fills the hole, a hole is created at that electron’s former location. Thus, holes behave as mobile positive charges in the valence band even though positively charged particles do not physically move in the material.
8. The hole-electron pairs formed in a semiconductor material by ionizing radiation are analogous to the ion pairs formed in a gas by ionizing radiation.
9. A crystal of a semiconductor material can be used as a radiation detector. A voltage is placed between two terminals on opposite sides of the crystal. When ionizing radiation interacts with the detector, electrons in the crystal are raised to an excited state, permitting an electrical current to flow, similar to a gas-filled ionization chamber. Unfortunately, the radiation-induced current, unless it is very large, is masked by a larger current induced by the applied voltage.
10. To reduce the magnitude of the voltage-induced current so that the signal from radiation interactions can be detected, the semiconductor crystal is “doped” with a trace amount of impurities so that it acts as a diode. The impurity atoms fill sites in the crystal lattice that would otherwise be occupied by atoms of the semiconductor material.
11. If atoms of the impurity material have more valence electrons than those of the semiconductor material, the impurity atoms provide mobile electrons in the conduction band. A semiconductor material containing an electron-donor impurity is called an n-type material (Fig. 17-8). N-type material has mobile electrons in the conduction band.
12. On the other hand, an impurity with fewer valence electrons than the semiconductor material provides sites in the valence band that can accept electrons. When a valence-band electron fills one of these sites, it creates a hole at its former location. Semiconductor material doped with a hole-forming impurity is called p-type material. P-type material has mobile holes in the valence band (Fig. 17-8).
13. A semiconductor diode consists of a crystal of semiconductor material with a region of n-type material that forms a junction with a region of p-type material (Fig. 17-9). If an external voltage is applied with the positive polarity on the p-type side of the diode and the negative polarity on the n-type side, the holes in the p-type material and the mobile conduction-band electrons of the n-type material are drawn to the junction. There, the mobile electrons fall into the valence band to fill holes. Applying an external voltage in this manner is referred to as forward bias. Forward bias permits a current to flow with little resistance.
14. On the other hand, if an external voltage is applied with the opposite polarity—that is, with the negative polarity on the p-type side of the diode and the positive polarity on the n-type side—the holes in the p-type material and the mobile conduction-band electrons of the n-type material are drawn away from the junction. Applying the external voltage in this polarity is referred to as reverse bias. Reverse bias draws the charge carriers away from the n-p junction, forming a region depleted of current carriers. Very little electrical current flows when a diode is reverse biased.
15. A reverse-biased semiconductor diode can be used to detect visible light and UV radiation or ionizing radiation. The photons of light or ionization and excitation produced by ionizing radiation can excite lower energy electrons in the depletion region of the diode to higher energy bands, producing hole-electron pairs. The electrical field in the depletion region sweeps the holes toward the p-type side and the conduction-band electrons toward the n-type side, causing a momentary pulse of current to flow after the interaction.
16. Semiconductor detectors are semiconductor diodes designed for the detection of ionizing radiation. The amount of charge generated by an interaction is proportional to the energy deposited in the detector by the interaction; therefore, semiconductor detectors are spectrometers. Because thermal energy can also raise electrons to the conduction band, many types of semiconductor detectors used for x-ray and gamma-ray spectroscopy must be cooled with liquid nitrogen.
17. The energy resolution of germanium semiconductor detectors is greatly superior to that of NaI(Tl) scintillation detectors. Liquid nitrogen-cooled germanium detectors are widely used for the identification of individual gamma-ray-emitting radionuclides in mixed radionuclide samples because of their superb energy resolution.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree