Although there are multiple variations in acquisition protocols for breast magnetic resonance (MR) imaging, there is agreement that components of high-quality technique include a bilateral acquisition obtained with a dedicated breast coil. Further, key pulse sequences should be included and spatial and temporal resolution should be sufficiently high to assess lesion morphology and kinetics. Artifacts must be recognized and avoided. The American College of Radiology Breast MRI Accreditation Program requirements provide minimum standards to guide facilities in technique. MR imaging at 3 T is increasingly available and offers signal-to-noise ratio advantages over 1.5 T but also some technical challenges
Key points
- •
High-quality breast magnetic resonance (MR) imaging that balances spatial and temporal resolution can be achieved at 1.5 T and higher magnetic field strengths, through understanding and application of key technical factors.
- •
Breast MR imaging at 3 T has the potential to improve image quality more than 1.5 T but also poses technical challenges, which should be addressed for quality image acquisition.
- •
The American College of Radiology Breast MRI Accreditation Program requirements for equipment and clinical images can guide appropriate acquisition protocols.
Introduction
Magnetic resonance (MR) imaging has increased in use over the past decade for detection and characterization of breast cancer. Evidence-based clinical applications of MR imaging for identification or assessment of breast cancer include evaluating patients with a new diagnosis of breast cancer, screening high-risk patients, monitoring response to neoadjuvant chemotherapy, and assessing patients with metastatic axillary adenocarcinoma of unknown primary cancer. Despite the increasingly widespread use of breast MR imaging, there is no standard protocol for image acquisition. Across the spectrum of potential breast MR imaging techniques, there is broad agreement that conventional breast MR imaging acquisition must use a dynamic contrast-enhanced (DCE) MR imaging protocol with adequate spatial resolution and temporal resolution. This strategy facilitates assessment of both morphologic and enhancement kinetic features, as are described in the standardized American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) MR imaging lexicon. Minimum requirements for breast MR imaging acquisition, including for equipment and clinical images, have now been established by the ACR Breast MRI Accreditation Program (BMRAP), and are discussed in detail below.
High-quality breast MR imaging is typically performed with at least a 1.5-T magnet to achieve adequate spatial and temporal resolution. However, breast MR imaging is increasingly being acquired at higher field strength of 3 T, because of the potential advantages of improved spatial resolution, improved contrast resolution, and decreased scan times. Although the move to higher magnetic field strength holds promise for improving breast MR imaging quality, additional technical, physical, and safety challenges posed by the 3 T technique must be addressed to realize these advantages.
Introduction
Magnetic resonance (MR) imaging has increased in use over the past decade for detection and characterization of breast cancer. Evidence-based clinical applications of MR imaging for identification or assessment of breast cancer include evaluating patients with a new diagnosis of breast cancer, screening high-risk patients, monitoring response to neoadjuvant chemotherapy, and assessing patients with metastatic axillary adenocarcinoma of unknown primary cancer. Despite the increasingly widespread use of breast MR imaging, there is no standard protocol for image acquisition. Across the spectrum of potential breast MR imaging techniques, there is broad agreement that conventional breast MR imaging acquisition must use a dynamic contrast-enhanced (DCE) MR imaging protocol with adequate spatial resolution and temporal resolution. This strategy facilitates assessment of both morphologic and enhancement kinetic features, as are described in the standardized American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) MR imaging lexicon. Minimum requirements for breast MR imaging acquisition, including for equipment and clinical images, have now been established by the ACR Breast MRI Accreditation Program (BMRAP), and are discussed in detail below.
High-quality breast MR imaging is typically performed with at least a 1.5-T magnet to achieve adequate spatial and temporal resolution. However, breast MR imaging is increasingly being acquired at higher field strength of 3 T, because of the potential advantages of improved spatial resolution, improved contrast resolution, and decreased scan times. Although the move to higher magnetic field strength holds promise for improving breast MR imaging quality, additional technical, physical, and safety challenges posed by the 3 T technique must be addressed to realize these advantages.
Technique requirements
High-quality breast MR imaging should include acquisition that is bilateral with complete coverage of the breasts and axillae, and obtained using a dedicated breast coil. The clinical images should include key pulse sequences, with appropriate spatial and temporal resolution for assessment of lesion morphologic and kinetic information, and be free of significant artifacts.
Bilateral Imaging
There are multiple advantages to routinely performing bilateral breast MR imaging. Clinically, bilateral imaging is highly desirable, because both breasts and axillae warrant evaluation in women undergoing MR imaging for high-risk screening or for extent of disease for a newly diagnosed breast cancer. Further, scanning both breasts allows important comparison of the symmetry of bilateral enhancement, which can help prevent diagnostic errors. It is particularly useful for establishing the pattern of background parenchymal enhancement (BPE), a benign physiologic phenomenon, and identifying masses or areas of nonmass enhancement that are sufficiently unique from BPE to warrant further evaluation. In addition, bilateral imaging is important for identifying benign-appearing circumscribed masses, which may be multiple and bilateral, such as intramammary lymph nodes and fibroadenomata. Unilateral axial plane acquisition is also less optimal from a technical standpoint, because it is more prone to wrap-around artifacts from the contralateral breast, because the phase-encoding gradient is typically applied in the right-to-left direction to minimize the effects of cardiac motion.
Coils
In order to maximize signal, breast MR imaging should be performed using only dedicated breast surface coils. Ideally, breast coils with a greater number of coil elements that allow for parallel imaging should be used. This requirement is because parallel imaging is particularly efficient for breast imaging, allowing reduced scan times or higher spatial resolution. Newer MR imaging systems available typically support up to 32 simultaneous radiofrequency (RF) channels. The breasts should be stabilized within the coil in the lateral to medial direction (for axial acquisitions) to minimize the effects of motion and its impact on subtraction images.
Contrast Agent
Breast MR imaging performed for cancer detection or characterization requires the administration of a gadolinium contrast agent. Chelated gadolinium has paramagnetic properties that result in decreased T1, T2, and T2* relaxation times. Thus, fluid-sensitive imaging, such as T2-weighted series, should be acquired before the administration of contrast. Because the decrease in relaxation from injection of the gadolinium chelate is greatest for T1-weighted sequences, DCE MR imaging is performed with T1 weighting. For breast imaging, the gadolinium chelate should be injected intravenously at a dose of 0.1 mmol/kg followed by a 20-mL saline flush at a rate of approximately 2 mL/s, using a power injector. This method both ensures contrast quickly reaches the intravascular space and allows for consistency in timing of contrast enhancement across examinations. Data do not support use of a higher dose of contrast, particularly because this could increase the extent of enhancement for BPE and benign discrete lesions as well as malignancies.
Spatial and Temporal Resolution
Breast MR imaging must meet basic spatial and temporal resolution requirements for appropriate diagnostic quality. High spatial resolution allows greater anatomic detail, enabling the interpreting radiologist to assess lesion morphology characteristics as well as potential disease involvement of the nipple-areola complex, skin, and chest wall. Temporal resolution (the speed at which dynamic postcontrast scans are obtained) determines the measured time-intensity curves or kinetic enhancement patterns over time. High spatial and temporal resolution scans are essential for a radiologist to identify and characterize breast lesions on breast MR imaging. However, these 2 factors have technological demands that must be balanced; achieving high spatial resolution requires a longer imaging time, and thus a relative trade-off in temporal resolution. As a result, optimally balanced breast MR imaging requires an understanding of the technical demands and clinical value of these intertwined acquisition factors. In our opinion, obtaining breast MR imaging scans at high spatial resolution is the most critical technical requirement for high-quality breast MR imaging, because morphologic features have been shown to provide the most influential information regarding probabilities of malignancy and thus BI-RADS assessments.
In order to observe some of the most specific MR imaging morphologic details, such as in masses the presence of spiculations (typically malignant) or dark internal septations (potentially benign), a spatial resolution of at least 3 mm through-plane should be achieved. Accordingly, the ACR BMRAP requires are for all multiphase precontrast and postcontrast T1-weighted images to be acquired with 3-mm slice thickness or less and a maximum in-plane pixel dimension of 1 mm 3 . Many morphologic details, such as spiculations, can be in the order of 1 mm in size. Such high spatial resolution (≤1 mm slice thickness) is difficult to achieve at 1.5 T without substantially compromising temporal resolution, but can be achieved at higher field strengths.
Temporal resolution is also an important factor in performing high-quality breast MR imaging. Typically, invasive breast cancers show early-phase rapid enhancement, with subsequent delayed-phase washout. Previous studies have shown that this early rapid enhancement occurs within 60 to 120 seconds after injection. Although opinions differ as to the optimal temporal resolution for DCE, an early postcontrast acquisition with sampling centered in the 60-second to 120-second period is reasonable, and can be achieved using varied methods dependent on whether a postcontrast delay is used before image acquisition, total duration of the postcontrast acquisition, and centering of k-space on the MR imaging unit. The ACR BMRAP requires that the first postcontrast imaging series be completed in 4 minutes or less from the time of completion of contrast injection to the end of the series acquisition. However, kinetic behavior depends on several factors, including histology, lesion grade, and microvessel density, resulting in a spectrum of enhancement curves for breast lesions. Although breast cancers may more frequently show early rapid and delayed washout enhancement than benign breast lesions, there remains substantial overlap in the kinetics of malignant and nonmalignant lesions of the breast. It has been suggested that higher temporal resolution achieved at greater field strengths may enhance the accuracy of kinetics for discriminating benign from malignant MR imaging findings. Furthermore, increased temporal resolution enables detailed quantitative pharmacokinetic analysis, which may provide more valuable information than conventional time-intensity curve analysis for lesion characterization and monitoring of treatment response.
With most modern MR imaging systems and their combinations of hardware and software, it is now possible to achieve both high spatial combined with appropriately high temporal resolution imaging. Further, hybrid techniques have been described that acquire high temporal resolution images (several acquisitions per minute) before and immediately after a high spatial resolution series.
Key Pulse Sequences
For optimal diagnostic usefulness, breast MR imaging should at minimum include a multiphase T1-weighted DCE series with precontrast, initial postcontrast, and delayed postcontrast images, as well as a T2-weighted or other fluid-sensitive series. Orthogonal plane reformatted images and maximum intensity projection images from the initial postcontrast series are also clinically useful. Because of the imaging speed required for breast MR imaging, gradient recalled echo (GRE) sequences should be used for the T1-weighted portions of the examination. The GRE pulse sequence should be spoiled to avoid any T2-like weighting effects or streaking artifacts. Spoiled GRE sequences can be performed with either two-dimensional (2D) or three-dimensional (3D) acquisition technique. 3D mode, in which a volume of multiple sections are obtained in a single excitation, is generally favored for breast imaging, because it allows higher signal-to-noise ratio (SNR) and greater T1 contrast. Because GRE sequences are not capable of providing true T2-weighted images, precontrast fluid-sensitive sequences require the use of spin echo, fast spin echo, or short T1 inversion recovery techniques.
Fat Suppression: Saturation and Subtraction
In order to ensure that lesions within the breast are not obscured by high signal related to fat within the breast, homogeneous fat suppression is essential. This suppression can be achieved at the level of image acquisition through fat saturation (also known as active fat suppression) techniques, which suppress the MR signal from fat, or at the postprocessing level by subtraction techniques.
Fat saturation can be achieved by canceling out the signal from fat through additional RF pulses or through selective water excitation. Using such techniques to actively suppress the fat signal incurs an acquisition time penalty, which could affect protocols that emphasize temporal resolution over spatial resolution. In addition, this technique also requires a homogeneous B 0 field over the entire field of view, which is more problematic in bilateral axial acquisition protocols. Some structures show high T1 signal not related to fat or gadolinium (eg, proteinaceous material or debris within ducts or masses and hemorrhage at sites of trauma, biopsy, or surgery), which can be difficult to discern from enhancement on images that use solely fat suppression.
In using subtraction techniques, data from the postcontrast T1-weighted images are subtracted from the precontrast T1-weighted images to create a similar visual effect of removing the signal from fat (as well as that from other nonenhancing structures with intrinsic high T1-weighted signal). However, the major drawback of such an approach is that even slight interscan patient motion can create artifacts that can simulate suspicious enhancement. This issue becomes even more pronounced in protocols that emphasize high spatial resolution, because small amounts of patient motion are more apparent in such scans. Therefore, care should be taken to maximize patient comfort and to immobilize the breast to limit this problem.
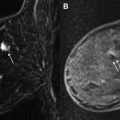
A combination of both fat saturation and subtraction techniques can be used in breast MR imaging protocols, which is our preferred approach. In such protocols, a precontrast T1-weighted series is obtained with fat saturation, followed by early and delayed dynamic postcontrast T1-weighted series, also with fat saturation. A fat subtraction series is made by subtracting the precontrast T1-weighted images with fat saturation from 1 of the postcontrast T1-weighted series with fat saturation (typically the first postcontrast series). This approach allows the interpreting radiologist to more efficiently evaluate for areas of true enhancement on the subtraction images by eliminating the complicating issue of high precontrast T1 signal. It also has the added benefit of allowing use of the source fat-saturated (but not subtracted) images in cases of significant interscan patient motion. Fig. 1 shows a case with both fat saturation and subtraction, with interscan motion causing artifactually suspicious enhancement on the subtraction series, clearly resolved as artifact on the source fat-saturated series.