Data support greater sensitivity of MR imaging compared with mammography and ultrasound in high-risk populations, in particular BRCA 1 and BRCA 2 carriers. Screening ultrasound improves cancer yield versus mammography alone in high-risk patients and in patients with dense breasts and is less expensive. Drawbacks include low positive predictive value, operator dependence, and significant physician time expenditure. Advances, such as refinement of automated whole-breast ultrasound, new outcomes data from ultrasound-detected masses in BI-RADS 3 and 4a categories, and development of new MR imaging sequences that allow rapid screening, potentially without use of contrast, will likely reveal the most appropriate tool over time.
Key points
- •
Data exist to support the greater sensitivity of MR imaging compared with mammography and ultrasound (US) in high-risk populations, in particular BRCA1 and BRCA2 carriers.
- •
Screening US has been shown to improve cancer yield compared with mammography alone in high-risk patients and/or patients with dense breasts and, relative to MR imaging, is inexpensive. It has drawbacks, however, that include low positive predictive value (PPV), operator dependence, and significant physician time expenditure.
- •
Advances, such as refinement of automated whole-breast US (AWBU), new outcomes data looking at US-detected masses in the Breast Imaging Reporting and Data System (BI-RADS) 3 and 4a categories (especially when computer-aided detection [CAD] and elastography are added), and the development and validation of new MR imaging sequences that allow rapid screening, potentially without use of contrast, will likely reveal the most appropriate tool over time.
Introduction
Breast cancer is a major cause of morbidity and mortality. It is anticipated that more than 290,000 women in the United States will be diagnosed with breast cancer in 2013. Early detection allows improved treatment options and survival for patients with breast cancer. Breast cancer screening is primarily performed with mammography, and annual screening is recommended for all women over 40 years of age. Mammography has proved a stalwart breast cancer screening tool, responsible for a significant portion of the 30% decrease in breast cancer mortality accomplished in the United States since 1990. Comparison of breast cancer mortality during prescreening and screening periods in 7 counties in Sweden (covering approximately 33% of the eligible female population) showed a 45% mortality reduction in populations who attended screening, with only a 12% reduction in mortality in women who did not undergo screening but had similar access to novel therapies and improved public awareness. Randomized controlled studies have confirmed a 31% mortality decrease in women aged 40 to 74 invited to screen, with the benefit confirmed on long-term follow-up.
Mammography, however, has well-recognized limitations. The false-negative rate of mammography is approximately 20% and may be higher in younger, premenopausal women and in those with dense breast tissue. The American Cancer Society (ACS) has identified factors associated with an increased risk for developing breast cancer: carriers of the BRCA1 and BRCA2 mutations (and untested relatives of BRCA1 and BRCA2 carriers); strong family history (2 or more close relatives with breast cancer or ovarian cancer, a first-degree relative with premenopausal cancer or more than 1 breast cancer, 1 or more relatives with both ovarian and breast cancers, or any male relative with breast cancer); prior mantle radiation between ages 10 and 30; prior breast biopsy showing a high-risk lesion (lobular neoplasia [LN] or atypical ductal hyperplasia); patients with mammographically dense breasts; and a personal history of prior breast cancer.
Some data suggest that mammography is a less-effective tool in some of these high-risk cohorts. Kriege and colleagues showed that among women with a familial or genetic predisposition to breast cancer, mammographic screening achieved 33.3% sensitivity, with only 12.5% of invasive cancers measuring less than or equal to 10 mm at time of detection. This compares unfavorably with established benchmarks showing a mammographic screening sensitivity of 84%, with 37.1% of invasive cancers measuring less than or equal to 10 mm, for an all-comers patient cohort. Tilanus-Linthorst and colleagues found that in their cohort of newly diagnosed breast cancer patients with BRCA1 and BRCA2 mutations, mammography was falsely negative in 62% of cases. Houssami and associates noted that screening mammographic sensitivity in patients with a personal history of breast cancer was significantly lower than a demographically matched non–personal history of breast cancer cohort (65.4% vs 76.5%, P <.001).
Perhaps most significantly, many studies have shown that screening sensitivity suffers in patients with mammographically dense breast tissue. Sensitivities ranging from 48% to 70% have been reported in screening patients with dense breasts, much lower than the 71% to 96% range for the general populations studied in Swedish and Canadian trials.
Recently, other imaging modalities, such as US and MR imaging, have been used in conjunction with mammography to add an additional layer of scrutiny, primarily for women at increased risk for developing breast cancer. Consequently, recommendations for breast cancer screening have become more complex. This article discusses the advantages and disadvantages of screening US and screening MR imaging, but it must be emphasized that these are adjunctive studies, not to replace but to supplement mammography, and that mammography is the only imaging modality that has been proved to decrease mortality from breast cancer.
Introduction
Breast cancer is a major cause of morbidity and mortality. It is anticipated that more than 290,000 women in the United States will be diagnosed with breast cancer in 2013. Early detection allows improved treatment options and survival for patients with breast cancer. Breast cancer screening is primarily performed with mammography, and annual screening is recommended for all women over 40 years of age. Mammography has proved a stalwart breast cancer screening tool, responsible for a significant portion of the 30% decrease in breast cancer mortality accomplished in the United States since 1990. Comparison of breast cancer mortality during prescreening and screening periods in 7 counties in Sweden (covering approximately 33% of the eligible female population) showed a 45% mortality reduction in populations who attended screening, with only a 12% reduction in mortality in women who did not undergo screening but had similar access to novel therapies and improved public awareness. Randomized controlled studies have confirmed a 31% mortality decrease in women aged 40 to 74 invited to screen, with the benefit confirmed on long-term follow-up.
Mammography, however, has well-recognized limitations. The false-negative rate of mammography is approximately 20% and may be higher in younger, premenopausal women and in those with dense breast tissue. The American Cancer Society (ACS) has identified factors associated with an increased risk for developing breast cancer: carriers of the BRCA1 and BRCA2 mutations (and untested relatives of BRCA1 and BRCA2 carriers); strong family history (2 or more close relatives with breast cancer or ovarian cancer, a first-degree relative with premenopausal cancer or more than 1 breast cancer, 1 or more relatives with both ovarian and breast cancers, or any male relative with breast cancer); prior mantle radiation between ages 10 and 30; prior breast biopsy showing a high-risk lesion (lobular neoplasia [LN] or atypical ductal hyperplasia); patients with mammographically dense breasts; and a personal history of prior breast cancer.
Some data suggest that mammography is a less-effective tool in some of these high-risk cohorts. Kriege and colleagues showed that among women with a familial or genetic predisposition to breast cancer, mammographic screening achieved 33.3% sensitivity, with only 12.5% of invasive cancers measuring less than or equal to 10 mm at time of detection. This compares unfavorably with established benchmarks showing a mammographic screening sensitivity of 84%, with 37.1% of invasive cancers measuring less than or equal to 10 mm, for an all-comers patient cohort. Tilanus-Linthorst and colleagues found that in their cohort of newly diagnosed breast cancer patients with BRCA1 and BRCA2 mutations, mammography was falsely negative in 62% of cases. Houssami and associates noted that screening mammographic sensitivity in patients with a personal history of breast cancer was significantly lower than a demographically matched non–personal history of breast cancer cohort (65.4% vs 76.5%, P <.001).
Perhaps most significantly, many studies have shown that screening sensitivity suffers in patients with mammographically dense breast tissue. Sensitivities ranging from 48% to 70% have been reported in screening patients with dense breasts, much lower than the 71% to 96% range for the general populations studied in Swedish and Canadian trials.
Recently, other imaging modalities, such as US and MR imaging, have been used in conjunction with mammography to add an additional layer of scrutiny, primarily for women at increased risk for developing breast cancer. Consequently, recommendations for breast cancer screening have become more complex. This article discusses the advantages and disadvantages of screening US and screening MR imaging, but it must be emphasized that these are adjunctive studies, not to replace but to supplement mammography, and that mammography is the only imaging modality that has been proved to decrease mortality from breast cancer.
Screening breast ultrasound
Background
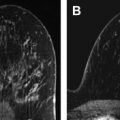
The appeal of using breast US for cancer screening was noted in the 1970s, related to its nondestructive technique. At that time, however, it was concluded that US lacked the spatial resolution to detect subclinical cancers ( Fig. 1 ). As a result, it was largely relegated to differentiating cystic from solid masses detected clinically or mammographically, at which it proved adept. As equipment improved, however, the role of breast US broadened, with evidence showing that it could be accurate in determining which solid masses might undergo short-term surveillance rather than requiring biopsy, assuming strict morphologic criteria were followed ( Fig. 2 ). As this practice became more widely accepted, the concept of using US as a screening tool to supplement mammography in women at increased risk and/or with dense tissue regained momentum.
Several studies subsequently validated the ability of whole-breast US (WBUS) to find small mammographically occult breast cancers, initially in patients referred for either a palpable lump or separate mammographic findings but eventually also in a screening setting. Kolb and colleagues showed that in women with dense breasts, screening WBUS increased the number of nonpalpable invasive cancers diagnosed by 42% compared with mammography alone and was especially beneficial in women under 50 years of age and in women with dense breasts. These studies were performed, however, with knowledge of the mammographic findings before performing WBUS. As Berg and colleagues pointed out, this leads to a bias in favor of WBUS. As a result, the need for a blinded trial of the efficacy of screening WBUS was identified. This need was realized with the American College of Radiology Imaging Network (ACRIN) 6666 study.
Multicenter Breast Ultrasound Screening Studies
ACRIN 6666, a prospective multicenter study, was designed to compare mammography alone to mammography plus US in a screening setting, using a cohort of patients at elevated risk for breast cancer ( Box 1 ) and heterogeneously or extremely dense breast tissue in at least 1 quadrant as determined by mammogram. In total, 2637 women across 21 sites formed the analysis population. Investigators were required to demonstrate proficiency in phantom scanning and pass mammography and breast US interpretive skills tests. They then evaluated mammographic and sonographic patient examinations independent of each other, in a blinded manner. Of the 40 cancers found, mammography identified 20, representing a yield of 7.6 per 1000; 12 cancers were seen on US alone, representing an impressive increased yield of 4.2 cancers per 1000 over mammography alone. Combined usage resulted in a yield of 11.8 per 1000 patients. Considering only the invasive cancers detected (5 of the cancers found by mammography alone were ductal carcinoma in situ [DCIS]; 1 DCIS was found on US alone), the median size of those found with mammography was 12 mm versus 10 mm for those found with US alone; 70% of invasive cancers identified with mammography were axillary node negative; and 89% of those found with US alone were node negative. Two additional multicenter studies have confirmed the results in ACRIN 6666, showing supplemental cancer detection yields of 4.2 to 4.4 per 1000.
- •
Personal history of breast cancer
- •
Model-based elevated risk
- ○
Lifetime risk greater than or equal to 25% (Gail or Claus model)
- ○
5-Year Gail model risk
- ▪
Greater than or equal to 2.5% or
- ▪
Greater than or equal to 1.7% and extremely dense breast tissue
- ▪
- ○
- •
Prior biopsy of high-risk lesion (ADH, ALH, LCIS, atypical papilloma)
- •
BRCA1 or BRCA2 positive
- •
Prior mantle radiation
Abbreviations: ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; LCIS, lobular carcinoma in situ.
Adding WBUS to a screening regimen, at least in women at increased risk and/or with mammographically dense breast tissue, thus can successfully and significantly improve cancer detection yield. The supplementally detected cancers tend to be small and node negative. Moreover, breast US does not use ionizing radiation. It is well tolerated by patients. US, as a breast imaging modality, is widely available. Compared with other technologies used for high-risk screening, it is cheap (approximately one-tenth the cost of breast MR imaging, for example) and does not require injection of contrast material. Why, then, has it not been widely and rapidly embraced by the breast imaging community?
Limitations of Ultrasound Screening
In addition to the good news regarding the additional cancers found when using supplemental US for breast cancer screening in elevated-risk populations, some bad news accompanied those results in the form of false-positive examinations. In ACRIN 6666, the false-positive rate for US alone was 8.1% (for mammography, 4.4%). The PPV2 for lesions found on US alone was 8.9% (for mammography, 22.6%). Short-term follow-up was recommended for 8.6% of patients (for mammography, 2.2%). This limitation persisted (as did the detection benefit), even on continued annual US screening (5% of women requiring biopsy, 7.4% PPV2 for US-only lesions), showing that it was not simply an issue of prevalence-examination–detected findings. Thus, the additional cancers found came with a price of unnecessary biopsies and added work-up.
There are other barriers to widespread acceptance of screening breast US. When a handheld technique is used, it is highly operator dependent: given its real-time nature, if a lesion is not detected during active scanning, it will be missed. In situ cancers often lack sonographic conspicuity. Real time US scanning is time intensive and labor intensive, which is especially of concern when requiring physician involvement. ACRIN 6666 was performed wholly using physician breast imaging specialists to scan the breasts. Reported average scan time per patient was 19 minutes for a bilateral examination, excluding time spent talking to patients, reviewing and reporting examinations, and comparing with prior examinations. Other studies have reported significantly faster screening scan times, ranging from 3 minutes and 59 seconds (patients had known normal mammogram) to 10 minutes (most of scans performed by a technologist alone). In this latter study, however, all abnormal findings were re-evaluated with repeat scanning by a fellowship-trained breast imager, presumably extending scan time. In addition, each of the studies notes a broad range of scan times, with longer scan times (up to 90 minutes in the ACRIN 6666 cohort) required in patients with large breasts, suspicious findings, or multiple findings. The ACRIN 6666 noted that added use of spatial compounding and color Doppler to evaluate all potentially suspicious findings likely added to the scan time. These tools are a vital part, however, of thorough lesion interrogation, especially when optimizing specificity is paramount. As a result, from personal experience, the authors think that the lengthier average scan time encountered in the ACRIN study is likely a reasonable expectation.
As a result of this required time intensivity, reimbursement becomes another issue confounding acceptance of WBUS screening. Global 2012 Medicare payments for bilateral digital mammography with CAD were $150.78 (professional component $37.78); for bilateral breast MR imaging, $716.83 (professional component $79.34); and for bilateral breast US, $99.39 (professional component $26.55). Mammography and breast MR imaging rarely require technical involvement by a radiologist, unlike breast US, where most breast imagers scan with or behind a technologist for completeness and confirmation, given the real-time nature of the technology. The prospect of adding a labor-intensive study with little resultant reimbursement, likely not enough even to cover the cost of the examination, may act as a disincentive to embracing screening US.
Future Directions
Despite its many advantages, before WBUS is likely widely accepted and implemented as a screening tool, its labor-intensive nature, operator dependence, and high false-positive rates need to be addressed.
Standardization of scanning technique and protocol as well as close attention to lesion feature analysis (within the context of the BI-RADS lexicon, American College of Radiology practice guidelines, and other evidence-based management algorithms), will allow greater confidence in lesion detection, characterization, and disposition. This may help to optimize throughput and intraobserver accuracy and enable outcomes research, with the hope of improving specificity. The ACRIN 6666 scan protocol was thorough and can serve as an excellent starting point for any center’s newly implemented screening practice. Some protocol components may be streamlined, however. For example, centers may find that it is not vital to routinely record negative images in each quadrant and the subareolar region. A single negative image per breast may suffice to document a negative examination. In addition, it may be determined that cysts (even the largest) do not need to be documented and eventually, if supported by the literature, that multiple benign-appearing masses do not require in-depth documentation. These modifications could lead to a decrease in scan time. Most breast imagers strongly support about hands-on involvement by a physician during scanning. Kaplan showed, however, that experienced technologists could be trained to successfully perform US screening. Although this training has to be validated within practice before adoption and is subject to personal preference, it represents another potential way to limit the physician time required to provide US screening.
Evolving technology may be the best hope for improving the efficiency and efficacy of US screening.
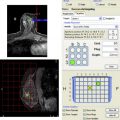
AWBU is being refined and has the potential to standardize and expedite study acquisition. It can be performed by a technologist without requiring physician involvement in scanning. Several vendors are pursuing a variety of unique acquisition and presentation methods using high-frequency probes: use of a robotically guided but standard transducer scanning the entirety of both breasts, with presentation of the images in a cine loop in a 2-D axial projection; use of a specialized large footprint transducer to image the central part of each breast with the patient supine and with presentation of the reconstructed images in the coronal plane ( Fig. 3 ); and use of a custom transducer to scan a prone, immersed breast, with presentation of 3-D reconstructed images. Although there is limited literature interrogating this new technology, some exists. Wang and colleagues showed that the diagnostic accuracy in differentiating benign from malignant lesions for AWBU is comparable to handheld US. A 2010 multicenter prospective screening study comparing mammography with AWBU screening showed that automated US screening resulted in an increase in cancer yield by 3.6 per 1000 compared with mammography alone. When the author of that study compared his automated screening technique results to ACRIN 6666 (handheld technique), he found that AWBU achieved a higher PPV (30.7% vs 8.9%) and found a higher percentage of less than or equal to 1.0-cm US-only detected invasive cancers (14.3% vs 6.2%). The recall rate was higher, however, than in ACRIN (7.2% vs 5.4%), which was not unexpected given that the scanning was physician performed in real time during ACRIN, allowing for immediate and definitive lesion evaluation. These results need to be validated by other studies. Some potential limitations of AWBU included its inability to scan the axillae (and with some systems, the periphery of the breasts) and difficulty with large breasts (deep lesions may not be well characterized). Specialized equipment adds to cost (estimated cost/examination by Kelly and associates: $300). CAD is being refined and evaluated as a way to further improve reader performance. Chabi and colleagues confirmed the results of other investigators in showing that sensitivity for cancer detection can be improved; they found that specificity suffered, however, especially among more experienced readers.
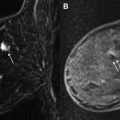
US elastography (USE) is an emerging technology that may improve lesion characterization and help reduce the false-positive biopsy rate seen with US screening. USE essentially evaluates the stiffness of tissue. Two types of USE are currently used clinically: compression elastography and shear wave elastography (SWE). By assessing strain (the degree to which tissue changes in shape, size, and position when external force, stress, is applied), the stiffness of a mass and its surrounding environs can be determined, which has implications about its likelihood of malignancy. When performing compression elastography, once a lesion is identified with real-time scanning, a software application allows the side-by-side display of an elastogram (a color-coded or gray-scale visual display of the semiquantitative strain data computed automatically), with required external compressive force applied simply by moderate transducer pressure ( Fig. 4 ). This elastogram is then qualitatively evaluated and can be assigned a score, as described by Itoh and associates : in their work, a lesion displaying uniform high strain (soft and malleable) receives a score of 1, and, at the other extreme, a lesion and its surrounding tissue showing low strain (firm and immobile) receives a score of 5. Much of the validation research surrounding compression elastography is based on comparing the performance of elastography scores with conventional B-mode–based BI-RADS assessment in characterization of masses seen with US. A meta-analysis of 5511 breast masses showed an improvement in specificity from 70% (B mode) to 88% (USE). USE alone, however, was less sensitive than conventional US (79% vs 96% for B mode), indicating that this technique cannot serve as a replacement for conventional US but rather as a tool that may allow safe deferral of biopsy of borderline suspicious (ie, BI-RADS 4a) lesions with an appropriate elastography score, thereby decreasing the false-positive rate of screening US ( Fig. 5 ). The semiquantitative nature of USE and, despite advances in equipment and software, its operator dependence and lack of reproducibility are among its limitations.