Chapter 5 Breast Ultrasound
Technical Considerations
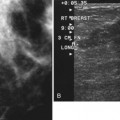
The American College of Radiology (ACR) has made specific recommendations for ultrasound labeling. The sonographer labels each finding according to its location in right or left breast, quadrant or clock position, scan plane (radial or antiradial, longitudinal or transverse), and number of centimeters from the nipple, along with the sonographer’s initials (Box 5-1). The sonographer takes images of the mass with and without measuring calipers. Any other pertinent clinical information, such as whether the lesion is palpable, may also be helpful to note.
Normal Sonographic Breast Anatomy
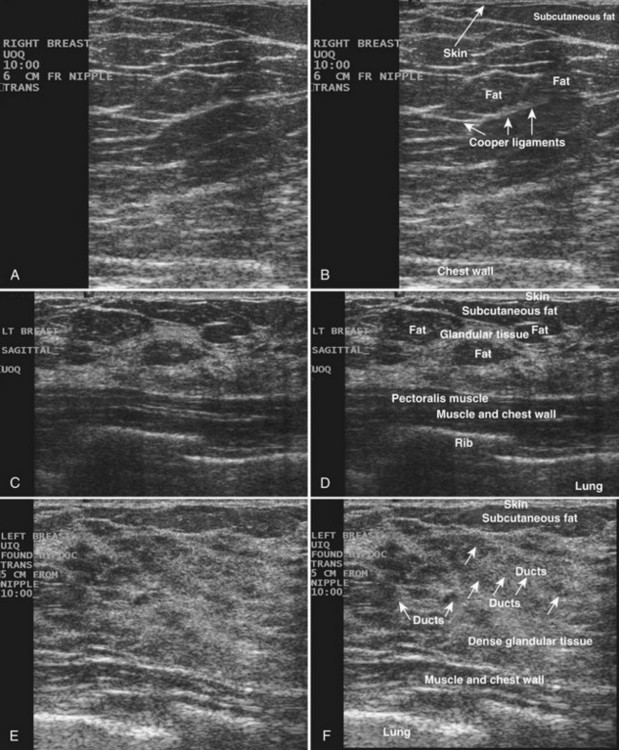
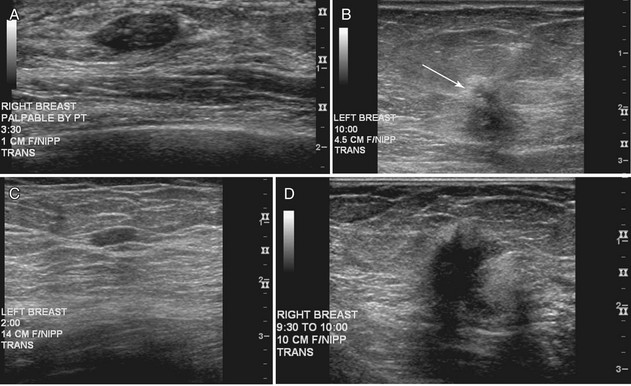
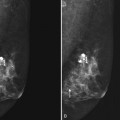
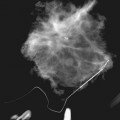
The breast is composed of fibrous connective tissue (Cooper ligaments) arranged in a honeycomb-like structure surrounding the breast ducts and fat (Fig. 5-1A and B). The proportion of supporting stroma to glandular tissue varies widely in the normal population and depends on the patient’s age, parity, and hormonal status. In young women, breast tissue is composed of mostly dense fibroglandular tissue. In later years, dense tissue involutes into fat in varying degrees, producing a mixed fatty and dense breast or an all-fatty breast (see Fig. 5-1C to F).
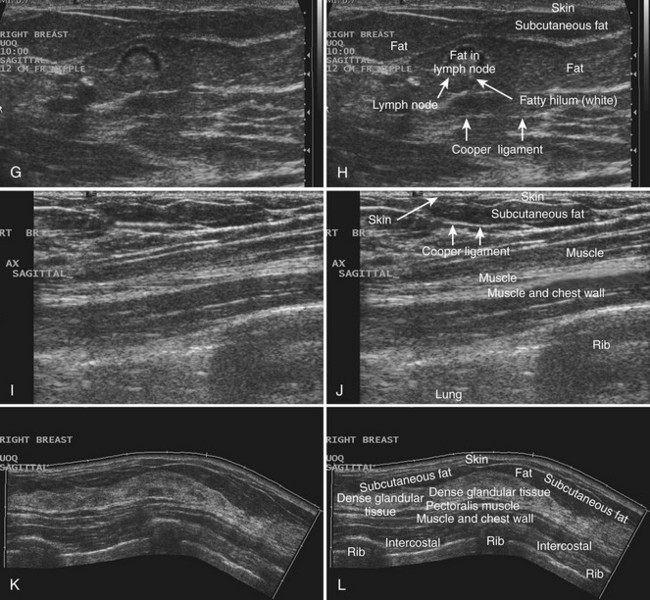
Breast tissues are either echogenic (white) or hypoechoic (black) on ultrasound. The skin is an echogenic line immediately under the transducer in the near field. It is normally about 2 to 3 mm thick and has a hypoechoic layer of dark subcutaneous fat immediately beneath it (Box 5-2). Unlike echogenic or white-appearing fat around the superior mesenteric artery in the abdomen, fat in the breast appears dark or hypoechoic. The only exception to hypoechoic fat in the breast is the echogenic fat in the middle of a lymph node. The normal lymph node is an oval, well-circumscribed mass with a hypoechoic cortex and fatty echogenic hilum, often seen in the upper outer quadrant and axilla, and often near an artery (see Fig. 5-1G and H).
Box 5-2 Normal Ultrasound Appearance of Breast Tissue*
Skin: 2- to 3-mm echogenic superficial line
Fat: hypoechoic (exception: fatty hilum in lymph nodes)
Breast ducts: hypoechoic tubular structures, oval in cross-section
Nipple: hypoechoic, can shadow intensely
Cooper ligaments: thin echogenic lines
Ribs: hypoechoic, round periodic structures at the chest wall
Breast glandular tissue and connective tissue are echogenic or white. Connective tissue has the highest acoustic impedance, fat has the lowest, and glandular parenchyma is of intermediate echogenicity. The Cooper ligaments are thin, sharply defined linear structures that support the surrounding fat and glandular elements (see Fig. 5-1I and J). Cooper ligaments in a fatty breast look like thin, white, gently curving lines surrounding hypoechoic fat. Normally, Cooper ligaments are thin and sharply demarcated. In breast edema, the fat becomes gray and the normally sharp Cooper ligaments become blurred.
The pectoralis muscle is a hypoechoic structure of varying thickness that contains thin lines of supporting stroma coursing along its long axis at the chest wall near the bottom of the image. The pectoralis muscle abuts the intercostal muscles and fascia of the chest wall (see Fig. 5-1K and L). Ribs in between the intercostal muscles are round or oval in cross-section, shadow intensely, and are seen at regular intervals along the chest wall. High-resolution transducers may display calcifications in the anterior portions of the cartilaginous elements of the ribs. Newcomers to breast ultrasound may mistake the ribs for masses, but their periodicity along the chest wall and the fact that one can palpate the ribs along their course will help prevent newcomers from making this mistake.
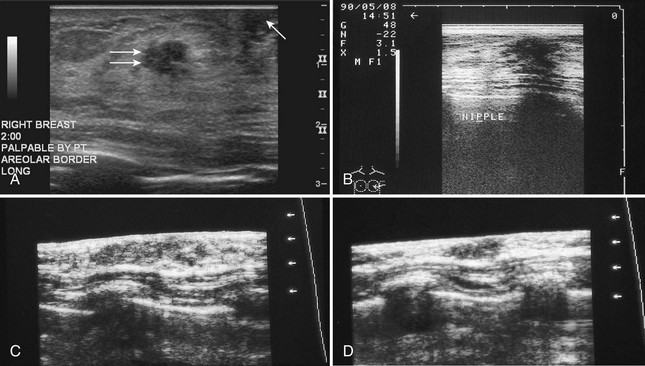
The nipple is a hypoechoic structure at the skin surface that occasionally produces an intense acoustic shadow as a result of the dense connective tissue within it (Fig. 5-2A and B). Because of the presence of retroareolar ducts and blood vessels, there may be marked vascularity in the retroareolar region on color or power Doppler imaging. Newcomers to breast ultrasound may mistake the nipple for a breast mass because of its hypoechoic appearance, shadowing, and the intense vascularity beneath it. However, knowledge of the shadowing, vascularity, and the ability to correlate the mass with the nipple on physical examination will help prevent newcomers from making this mistake.
In children, the breast bud that develops into the adult breast is right underneath the nipple. The breast bud may produce an asymmetric lump under the nipple that may be mistaken for a mass rather than a normal developing structure (see Fig. 5-2C and D). This normal structure should be left alone because surgical removal of the breast bud results in no breast formation on the ipsilateral side.
Ultrasound Evaluation of Mammographically Detected Findings
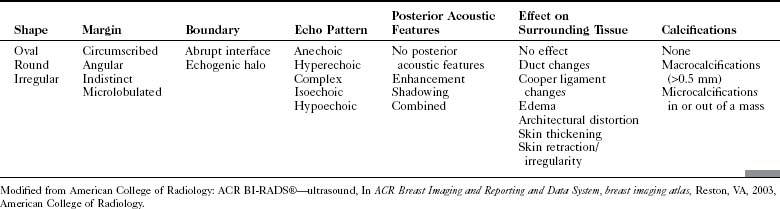
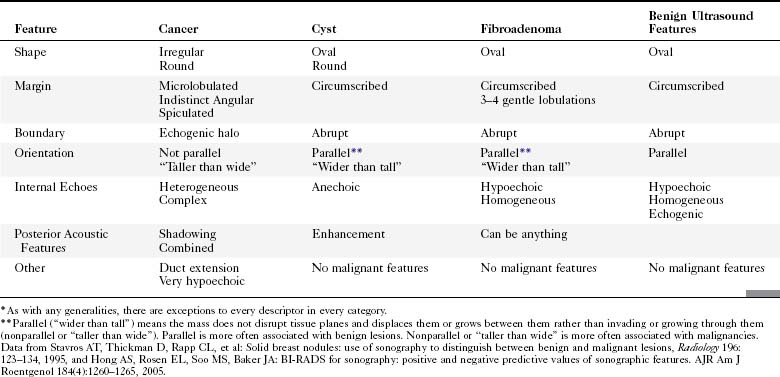
The ACR Breast Imaging Reporting and Data System (BI-RADS®) committee developed an ultrasound lexicon to provide descriptors for findings seen by ultrasound and recommended specific descriptors for breast masses (Table 5-1). Use of the words in the ACR BI-RADS® lexicon helps clarify one’s impression of the finding, improves communication between the radiologist and referring physician, and may trigger specific patient managements. This is because specific ultrasound features described by the lexicon suggest either benign masses or cancer. Although there is some overlap in benign versus malignant ultrasound features, the radiologist can use the lexicon to be reminded of what features should be searched for on the image (Table 5-2).
The BI-RADS® ultrasound lexicon descriptors for breast masses and their effect on the surrounding breast tissue are illustrated in Figures 5-3 to 5-6. Mass shapes are reported as oval, round, or irregular. Mass margins are circumscribed, angular, indistinct, microlobulated, or spiculated. The internal echo pattern is described as anechoic (all black inside), hyperechoic (white), complex (mixed black and white), isoechoic (equal), or hypoechoic (dark). Posterior acoustic features are described as no posterior acoustic features, enhancement (white), shadowing (dark), or a combined pattern. The boundary between the mass and the surrounding tissue is described as having an abrupt interface or as containing an echogenic halo (a white blurry band surrounding the mass). Calcifications are described as no calcifications, macrocalcifications (>0.5 mm), microcalcifications within the mass, or microcalcifications outside the mass. Effects of the mass on surrounding breast tissue are described using the terms no effect, duct changes, changes in Cooper ligaments, edema, architectural distortion, skin thickening, skin retraction, and skin irregularity.
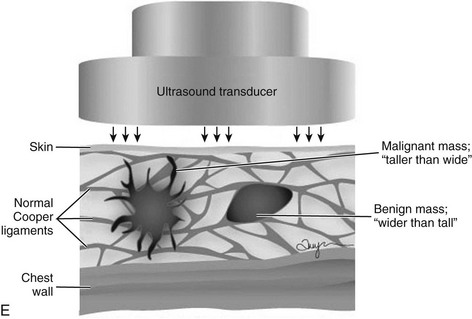
The terms parallel or not parallel relate to tumor growth patterns with respect to normal tissue planes. They are important because they indicate if the mass is growing along or in between tissue planes versus growing through them. A parallel growth pattern indicates a benign finding (wider than tall, as described by Stavros and colleagues) because it indicates a growth pattern along tissue planes. Not parallel or taller than wide indicates that the mass is growing through the normal tissue planes, which is not normal and indicates cancer (see Fig. 5-6E).
Finally, the ultrasound BI-RADS® lexicon suggests standard reporting for masses, as in Box 5-3.
Box 5-3 Ultrasound Mass Reporting
Breast Cysts, Intracystic Tumors, and Cystic-Appearing Masses
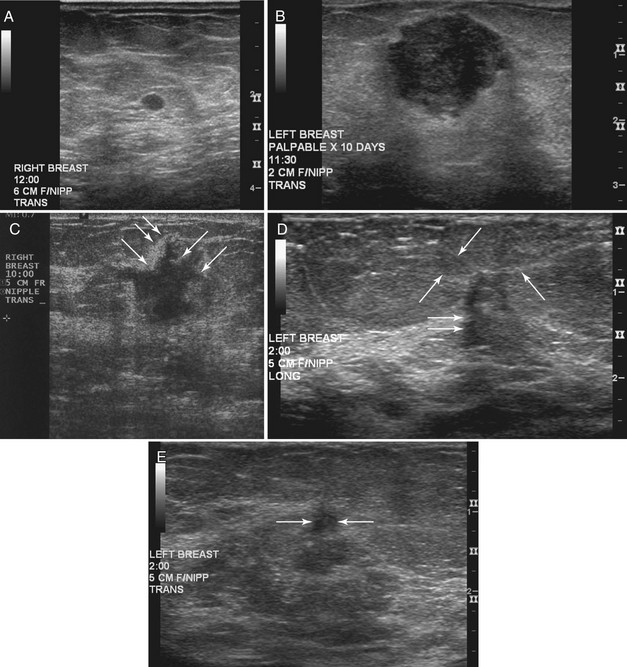
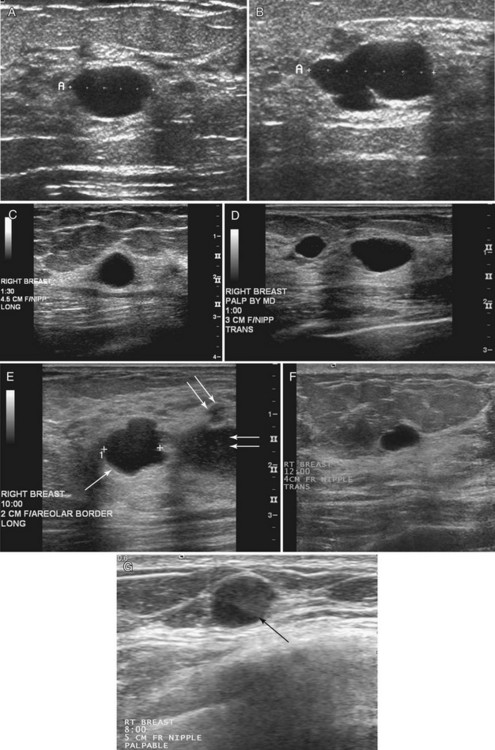
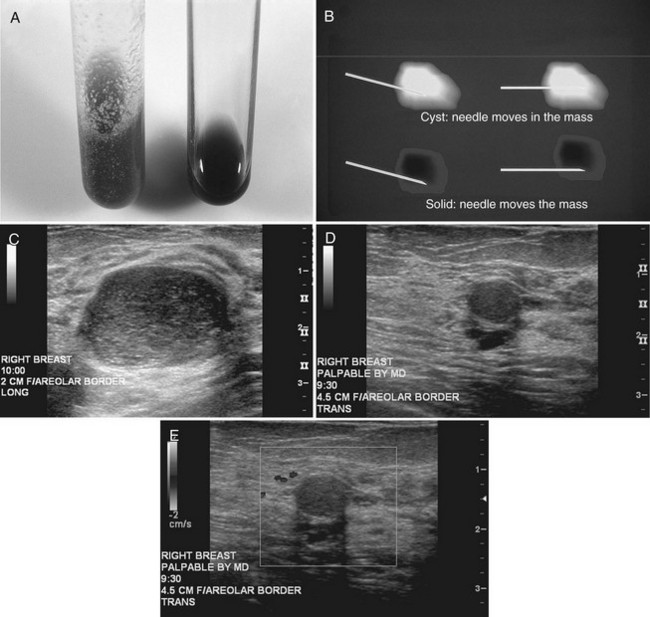
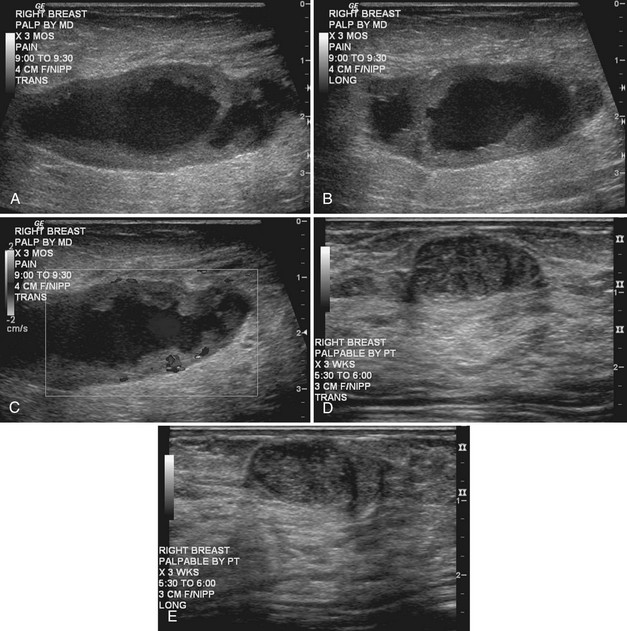
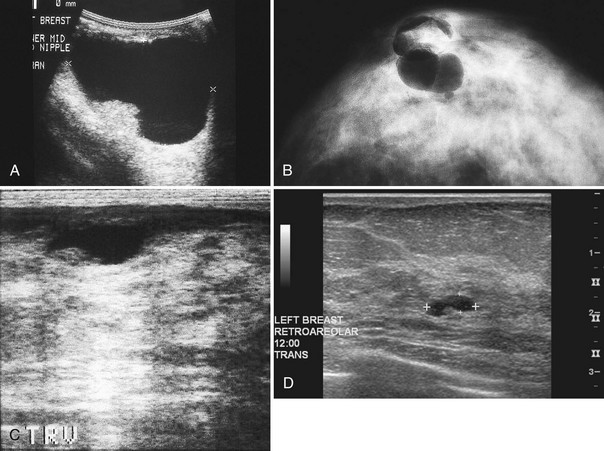
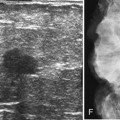
Strict ultrasound criteria for a simple cyst include a mass with well-circumscribed margins, sharp imperceptible anterior and posterior walls, a round or oval contour, absence of internal echoes, and posterior acoustic enhancement (Box 5-4 and Fig. 5-7A). Cysts may be single or multiple, gathered into small clusters, or contain thin septations (see Fig. 5-7B to D). Cysts are not malignant or premalignant, but examination of them is important because they may cause lumps that mimic round cancer on physical examination or mammography. When palpable, a cyst is a smooth, mobile mass on physical examination. Occasionally cysts appear as a visible mass if the patient is supine and the cyst is large. Cysts may be painful and may wax and wane with the patient’s menstrual cycle. If a mass is proven to be a cyst by ultrasound, the patient can be monitored by screening mammography because cysts are not cancer. Symptomatic cysts that are painful or cause a lump that disturbs the patient can be treated by aspiration. Cysts may be simple or “complicated,” meaning that the cyst contains sloughed debris. These complicated cysts contain material within them rather than being anechoic. Some complicated cysts require aspiration to confirm that they are cysts rather than solid masses (see Fig. 5-7E).
Deeply located cysts may not show enhanced through-sound transmission because of their location close to the chest wall, and lateral cyst walls may be obscured by refractive shadows (see Fig. 5-7F). These problems may be resolved by repositioning the patient or the transducer to scan from a different angle. This permits visualization of distal acoustic enhancement or eliminates the refractive shadows obscuring the sharp cyst walls. Acorn cysts contain a fluid/fluid level, with the dark dependent portion of the cyst representing clear fluid (the acorn) and the lighter top representing layering fluid above it (the acorn cap) (see Fig. 5-7G). Changing the patient’s position may cause the layer to move dependently, clinching the diagnosis of an acorn cyst.
The internal characteristics of cysts must be analyzed to exclude mural masses or irregular thick walls, which indicate complex masses. Complex masses contain cystic and solid components; intracystic tumors and necrotic neoplasms are in the differential diagnosis. Complex masses are different from complicated cysts, which contain debris. Complex masses might be cancer, but complicated cysts are benign (Table 5-3 and Boxes 5-5 and 5-6).
Table 5-3 BI-RADS® Ultrasound Special Cases (Cystic)
| Cystic Mass Type | Description | Differential Diagnosis |
|---|---|---|
| Clustered microcysts | Benign | |
| Complicated cysts | Benign | |
| Complex mass | Has cystic and solid components |
Modified from American College of Radiology: ACR BI-RADS®—ultrasound, In ACR Breast Imaging and Reporting and Data System, breast imaging atlas, Reston, VA, 2003, American College of Radiology.
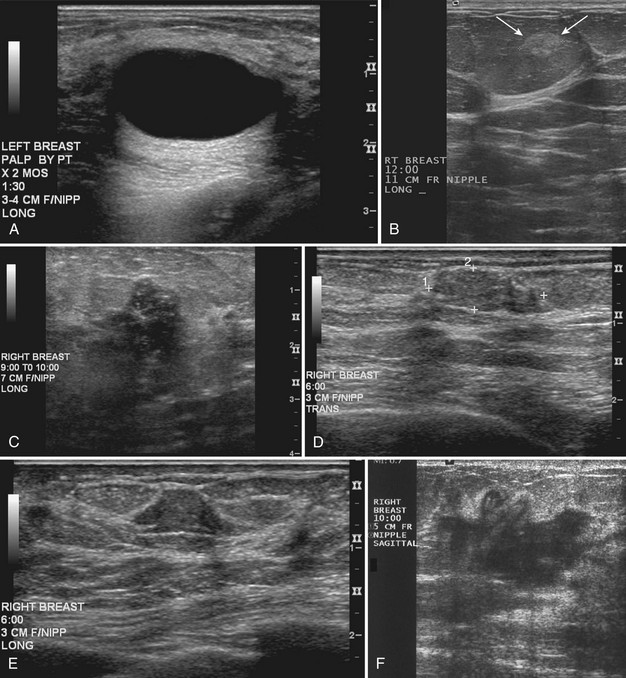
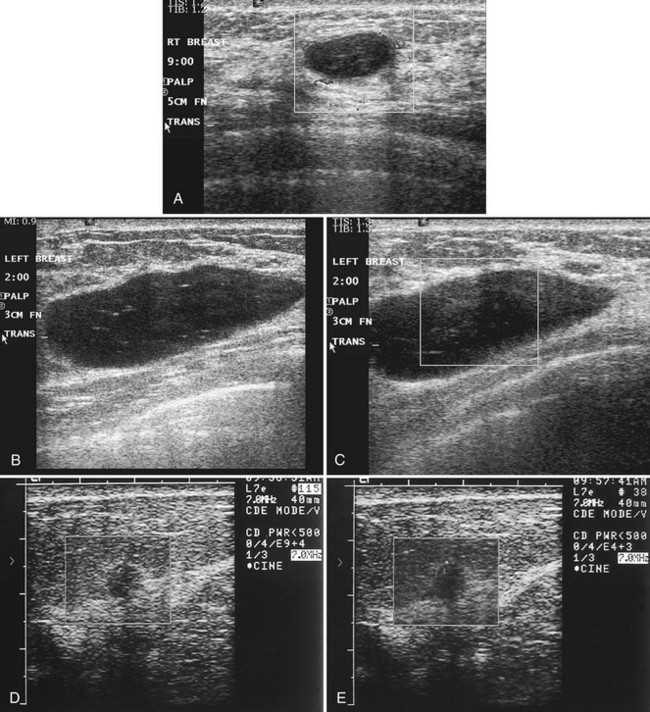
Real-time ultrasound imaging can help distinguish speckle artifact from debris in cyst fluid from a solid mass. Real-time ultrasound can show particulate matter slowly moving inside the cyst. On real-time imaging, the debris causes speckle artifact, which swirls in the cyst like fake snow in a snow globe (Fig. 5-8A to C). Placing the patient in the decubitus position can cause a difference in the sedimentation pattern in the complicated cyst, but not always. Color Doppler or power Doppler ultrasound can detect movement of particulate matter within complicated breast cysts or blood vessels in solid masses (see Fig. 5-8D and E). Doppler imaging will show no blood vessels in breast cysts. Unfortunately, the absence of blood flow in a mass is not diagnostic of a cyst because Doppler imaging does not always detect blood flow in solid masses or even in cancers.
Differentiation of complicated cysts from benign or malignant cystic masses can be tricky (see Table 5-3). Some cysts contain true internal echoes as a result of thick tenacious fluid or hemorrhage from previous aspirations. Some cysts have thick walls as a result of inflammation from cyst fluid leaking into the surrounding tissues. In cases in which all the sonographic criteria of a simple cyst have not been met, fine-needle aspiration may obviate the need for core needle biopsy or surgical biopsy. Once the needle is within the mass, the presence of cyst fluid rather than solid tissue can be confirmed by moving the needle, as suggested by Stavros and colleagues (Fig. 5-9). Cysts that do not fulfill all criteria for simple cysts, in the right clinical setting, require aspiration (see Fig. 5-9C to E). Cyst fluid should be sent for cytologic analysis if it is bloody, if there is an intracystic mass on ultrasound or pneumocystography, or if the patient has had prior intracystic carcinoma. Clear cyst fluid can be discarded if there are no clinical factors that would require cytologic examination.
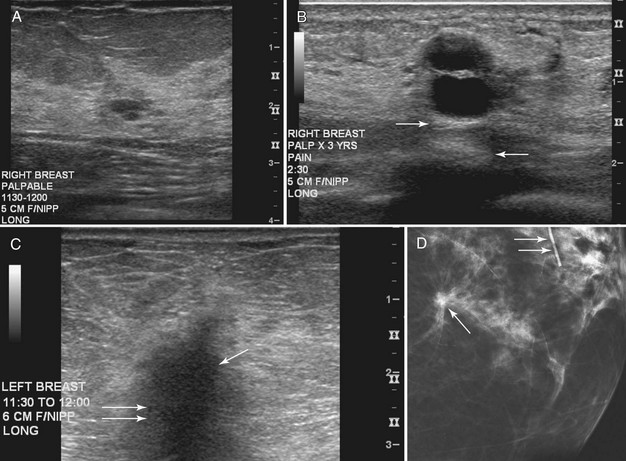
On the other hand, complex cystic masses (i.e., fluid-filled masses with thick walls or mural projections) require biopsy to exclude the rare intracystic papilloma, intracystic carcinoma, phyllodes tumor with a marked cystic component, or solid cancers with central necrosis. Other complex masses include hematoma, abscess, galactocele, and seroma; management of these masses is based on their appearance and the clinical situation (Fig. 5-10).
Intracystic carcinomas are a rare subgroup of tumors that arise from the walls of a cyst; they represent 0.5% to 1.3% of all breast cancers (Fig. 5-11A). These tumors have a better prognosis than other malignant breast neoplasms do. On ultrasound, intracystic carcinomas often appear as solid mural excrescences projecting into the cyst fluid. Differentiation of intracystic carcinoma from benign intracystic papilloma is not possible, and surgical biopsy is thus necessary. The finding of a mural nodule within a cyst has the differential of an intracystic carcinoma, papilloma, a cyst with debris, and reverberations in a simple cyst produced by high gain settings (see Fig. 5-11B to D). Color or power Doppler imaging may be helpful if a blood vessel can be identified in the intracystic mass.
Benign Solid Masses: Fibroadenoma and Fatty Pseudolesions
On ultrasound, Cole-Beuglet and colleagues describe typical fibroadenomas as solid masses with well-circumscribed, round or oval borders and containing weak low-level homogeneous internal echoes with enhanced, decreased, or unchanged sound transmission. Stavros and colleagues and Fornage and colleagues have described fibroadenomas as smooth, wider than tall solid masses. Stavros and colleagues further characterize fibroadenomas as having at most four gentle lobulations and homogeneous internal echo texture (Box 5-7 and Fig. 5-12A to C).
Box 5-7
Benign Mass Characteristics
Ellipsoid shape (wider than tall)
Four or fewer gentle lobulations
Intense homogeneous hyperechogenicity (in comparison to fat)
From Stavros AT, Thickman D, Rapp CL, et al: Solid breast nodules: use of sonography to distinguish between benign and malignant lesions, Radiology 196:123–134, 1995.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree