, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
A minimum basic knowledge regarding cardiac embryogenesis is necessary to facilitate understanding congenital heart malformations. This chapter is meant as a basic overview to achieve this end and is not meant to fully encompass the totality of this complex subject.
A minimum basic knowledge regarding cardiac embryogenesis is necessary to facilitate understanding congenital heart malformations. This chapter is meant as a basic overview to achieve this end and is not meant to fully encompass the totality of this complex subject.
The human embryo at the primitive streak stage (about 15 days after fertilization) is morphologically symmetrical [1]. The primordia of the cardiovascular system originate as clusters of paired, symmetrical mesenchymal cells in the coelomic mesoderm. These cells migrate and multiply and in some cases resorb to ultimately form the mature human heart. Errors in this process lead to congenital heart defects.
Initially located on the cephalad and dorsal aspect of the embryo, the mesenchymal cells migrate around the buccopharyngeal membrane of the forming foregut and join at the midline of the ventral aspect of the embryo. Subsequent infolding and fusion of primitive tissue along its long axis transforms a flat structure into a tubular shape. Initially, the cardiovascular primordia lie within the cephalad section of the undivided coelomic cavity. The right and left intracoelomic cavities approach the midline and join together, forming a midline thoracic cavity (the pericardium), which surrounds the primitive heart [1–3].
At first, the primitive heart is a straight median tube called the straight tube heart (Fig. 1.1a). The arterial and venous ends are relatively fixed in space requiring that the growth of the bulboventricular segments occur by bending of the cardiac tube. Soon, the primitive cardiac tube develops constrictions which define four future segments: atria, ventricle, bulbus cordis, conus, and truncus arteriosus (Fig. 1.1b) [2–4]. The cranial-most area is the bulbus conus, which connects cranially with the truncus arteriosus, which in turn connects to arterial structures (aortic arches and the dorsal aorta). Caudal to the bulbus cordis is the primitive ventricle. The caudal-most structure of the primitive heart is the primitive atrium, which connects to the sinus venosus, which in turn connects to the omphalomesenteric veins. At day 21–23, all structures are connected in series. There is no inner circulation, and the heart is simply a hollow, empty tube. The heart tube starts to beat on day 22, but circulation does not begin until days 27–29 [2–4].
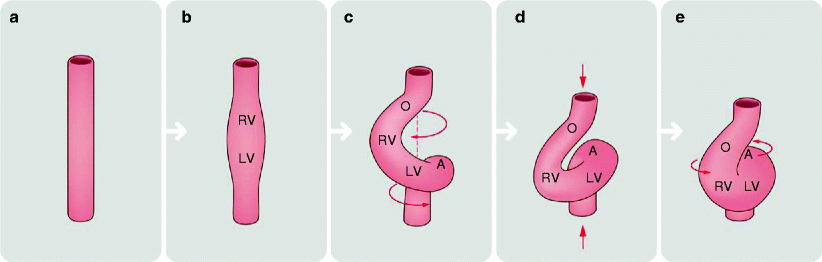

Fig. 1.1
Looping of the primitive heart tube. Straight heart tube (a) curves ventrally (b) and twists around its craniocaudal axis to form a C-shaped loop (c). Subsequently, the distance between its cranial and caudal ends shortens (d) and the loop untwists with the ventral and leftward shift of the outflow tract, ventral shift of the primitive right ventricle and rightward shift of the atrioventricular canal (e). RV embryonic right ventricle, LV embryonic left ventricle, O common outflow tract, A common atrium
The caudal-most areas, the primitive atrium and sinus venosus, are the primary determinants of atrial sidedness (situs). The atria are fixed in position early in development by the sinus venosus and its entering veins [5]. Errors in the early stage of heart tube development will result in abnormalities of atrial position (situs). Since atrial situs corresponds to visceral situs, abnormalities of atrial situs are often associated with abnormalities in the situs of other organs.
1.1 Ventricular Development and Cardiac Looping
The primitive heart tube continues to develop by continuous cellular migration and multiplication. On approximately day 23, the embryonic heart becomes morphologically asymmetric due to the right or left looping of the bulboventricular segments, forming either a rightward loop (dextra or D-loop) or a leftward loop (levo or L-loop), respectively (Fig. 1.1c) [6]. This process likely occurs due to differential migration and multiplication of primordial cardiac cells.
The bulbus cordis produces the morphologic right ventricle while the morphologic left ventricle is formed from the ventricle of the bulboventricular loop. Thus, the direction of the initial cardiac loop determines the eventual ventricular locations. During the looping process, the orientation of the heart changes from an anterior/posterior orientation to a left/right orientation and irreversibly establishes the relationship between the ventricles and the already-determined situs of the atria.
It should be noted that the bulboventricular looping (rightward or leftward) is independent of the process that determines the relative atrial positions (situs). While the atrial sidedness is determined by processes that determine visceral situs, the anatomical relationships between the ventricles and the aortic and pulmonary trunks are decided by the looping process. In D-looping, the bulbus cordis (future morphologic right ventricle) is to the right of midline and the bulboventricular segment (future morphologic left ventricle) is to the left. Conversely, in L-looping, the bulbus cordis (future right ventricle) is to the left of the bulboventricular segment (future left ventricle). D-loop is the normal (solitus) cardiac loop and L-loop is a mirror image (inversus) loop.
As the heart tube loops, the bulboventricular segment acquires a U shape, causing the atrium and sinus venosus to become dorsal structures (Fig. 1.1d) [2].
Additionally, the looping pattern of the bulboventricular segments determines the irreversible relationship between the fourth and the sixth aortic arches which ultimately form the distal aortic and pulmonary trunks. Thus, bulboventricular looping patterns permanently determine the anatomic relationship between the aortic and pulmonary trunks. In D-looping with normal development, the pulmonary artery is located anteriorly, superiorly, and to the left of the aorta. In L-looping, the pulmonary artery is located anteriorly, superiorly, and to right of the aorta.
Figure 1.2 depicts D-looping versus L-looping.
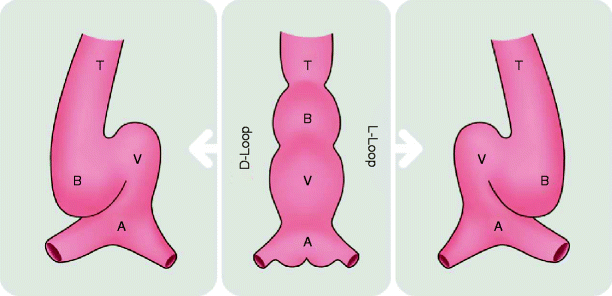

Fig. 1.2
Diagram illustrates looping (bending) of the primitive cardiac tube. The cardiac tube is depicted from anterior view. The cardiac tube is comprised of the atrium (A), ventricle (V), bulbus cordis (B), and truncus arteriosus (T). The cardiac tube normally bends to right, forming a D-bulboventricular loop (D-loop). Rarely, the tube may bend leftward, forming L-bulboventricular loop (L-loop)
Alterations in looping patterns can lead to four basic morphologic combinations: situs solitus with D-looping, situs solitus with L-looping, situs inversus with D-looping, or situs inversus with L-looping. Situs solitus refers to the correct sidedness of the morphologic atria and inversus refers to incorrect morphologic atrial sidedness. D-looping refers to correct ventricular sidedness and L-looping refers to a reversal of the morphologic ventricular sidedness.
In the normal situation, situs solitus with D-looping, the morphologic atria are on their respectively correct sides (right atrium on the right, left atrium on the left) and the morphologic ventricles are also on the correct side such that the morphologic right ventricle connects with the right-sided atrium and the morphologic left ventricle connects with the left-sided atrium. In situs solitus with L-looping, the atria are again on their respectively correct sides but ventricles are reversed. The morphologic right ventricle (now anatomically left sided) connects to the left-sided atrium and the morphologic left ventricle (now right sided) connects to the right-sided atrium. In situs inversus with D-looping, the morphologic atria are reversed (right atrium on the left and left atrium on the right) but the ventricles are on their respectively correct sides. The morphologic right atrium (now anatomically left sided) connects to a morphologic left ventricle (left sided), and the morphologic left atrium (right sided) connects to a morphologic right ventricle (right sided). In situs inversus with L-looping, the atria are again reversed and the ventricles are also reversed. The morphologic left atrium (now right sided) empties into a morphologic left ventricle (right sided), and the morphologic right atrium (now left sided) empties into a morphologic right ventricle (left sided) [7].
Abnormalities in looping may also lead to ventricular and great artery transformations.
Table 1.1 depicts the various situs and looping combinations.
Table 1.1
Depiction of the various situs and looping combinations