John D. Grizzard, MD
Navneet Narula, MD
Jonathan W. Weinsaft, MD
CARDIAC NEOPLASMS
PATIENT HISTORY
A 45-year-old woman presents for evaluation in the context of progressive dyspnea. Prior medical evaluation is significant for a mobile left atrial (LA) mass identified on screening echocardiography (echo). Further cardiac assessment is being considered via cardiac magnetic resonance (CMR) or computed tomography (CT) imaging.
GENERAL PERSPECTIVE
Evaluation of known or suspected cardiac masses is a common indication for CMR and CT. Masses can present with a broad array of symptoms or can be clinically silent, and the work-up may be prompted only by incidental findings on other tests such as ECHO. As clinical features can overlap, imaging can be useful to distinguish between diagnostic possibilities. Both CMR and CT provide highresolution assessment of location, morphology, and tissue characteristics of cardiac masses. These features can help to distinguish between neoplasms and thereby aid in diagnostic evaluation.
The following sections stratify cardiac masses into 4 general categories—benign neoplasms, malignant neoplasms, thrombus, and pseudo-masses (ie, normal anatomic structures). For each category, specific diagnoses are associated with key epidemiologic, clinical, and imaging features that can be used to aid in diagnostic evaluation.
BENIGN NEOPLASMS
Approximately three-fourths of primary cardiac neoplasms are benign.1 In addition to epidemiologic and clinical differences, these can be distinguished from one another based on tissue composition. From an imaging standpoint, differences in tissue composition can produce variable tissue characteristics on both CMR and CT. Tissue characterization imaging has been shown to provide incremental utility as compared to conventional approaches that focus on location or morphology of cardiac neoplasms.2,3 The following section details leading diagnostic considerations for benign cardiac neoplasms, with particular focus on associations between actual tumor composition and findings on tissue characterization imaging.
DIFFERENTIAL DIAGNOSIS
Myxoma
Epidemiology
Myxomas represent the most common primary benign cardiac neoplasm, constituting approximately 50% of such cases. Myxomas can be sporadic or familial. Sporadic myxomas occur more often in females (1.5-3:1) and typically present in adulthood.4 Approximately 10% of cases are familial, the majority of which are associated with Carney complex, a condition that manifests in an autosomal dominant pattern and has been linked to 17q23-q24 or 2p16 chromosomal mutations.5 As compared to sporadic cases, familial myxomas generally present at a younger age and are more likely to recur following surgical resection.
Clinical Features
Myxomas can be clinically silent, nonspecific in presentation, or manifest with a classic triad—constitutional symptoms (fever, malaise), mitral valve (MV) obstruction (mimicking mitral stenosis), and embolic phenomena. In patients with Carney complex, familial cardiac myxomas are often associated with dermatologic myxomas, dermal hyperpigmentation (lentigenosis), and endocrinopathies (adrenocortical hyperplasia, pituitary hyperplasia).
Etiology/Tissue Composition
The histological diagnosis of myxoma is based on identification of cardiac myxoma cells in a myxoid background. Grossly, myxomas typically have a gelatinous appearance due to abundance of myxoid matrix.
Imaging Findings
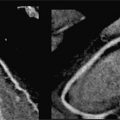
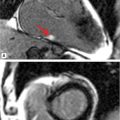
• Location: Myxomas are typically located in the LA (75%-80%) with a stalk-like attachment to the fossa ovalis (Figure 10-1). They are less often located in other cardiac chambers (right atrium [RA], left ventricle [LV], or right ventricle [RV]).6 Familial myxomas are more likely to be multifocal and/or located outside the LA.
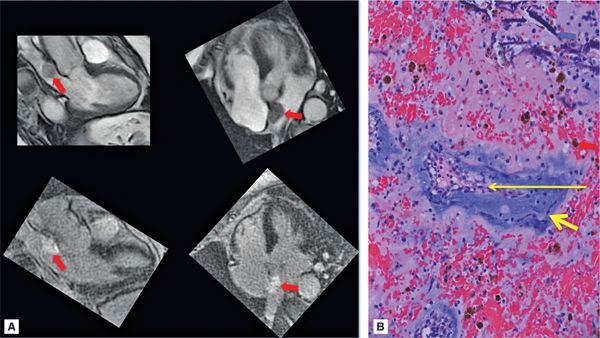
FIGURE 10-1 Left atrial Myxoma. (A) Cine-CMR (top), shown in 3- and 5-chamber (CH) orientation, demonstrates typical morphologic appearance of a LA myxoma. Note the mobile lobulated mass within the LA (red arrow), partially adherent to the interatrial septum. DE-CMR (bottom) demonstrates the mass to exhibit marked contrast uptake, consistent with prominent vascular supply. (B) On histopathology (hematoxylin and eosin stain, magnification 20X), a diagnostic feature of cardiac myxoma is the presence of myxoma cells (thick yellow arrow) that form rings around capillaries (thin yellow arrow). Note hemosiderin laden macrophages (red arrow) and degenerative changes including calcium (blue arrow).
• Morphology: Myxomas classically appear as a lobulated mobile mass attached to a narrow-based stalk. Cine-CMR or cine-CT (ie, retrospectively ECG gated throughout the cardiac cycle) can demonstrate mobility pattern and evaluate for dynamic protrusion through neighboring cardiac valves.
• Tissue characteristics: The imaging appearance of myxomas reflects the complex histologic composition of these neoplasms. Myxomas often have a well-defined stalk where they attach to the fossa ovalis or myocardium, which is relatively well vascularized and may enhance on perfusion imaging or manifest central enhancement on postcontrast delayed-enhancement (ie, DE-CMR) imaging (Figure 10-2). The globular periphery of the lesion most often has a gelatinous, myxoid composition and lacks a significant soft tissue component, which results in a relatively avascular appearance on perfusion or delayed-enhancement imaging. It is important to note that myxoma-associated vascularity can vary and that some cases may not show evidence of a discrete tumor stalk (ie, central enhancement) but instead demonstrate diffuse contrast uptake on imaging (Figure 10-1).
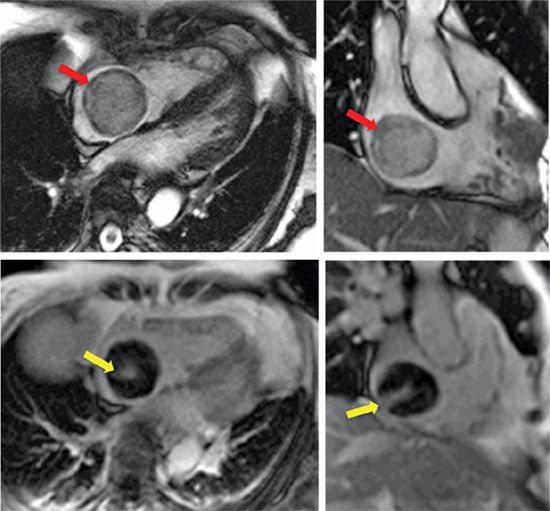
FIGURE 10-2 Right atrial Myxoma. right atrial myxoma assessed using CMR. Cine-CMR (top), shown in 4-CH and dedicated right ventricular orientations, demonstrates a large circumferential RA mass (red arrow) that dynamically protrudes through the tricuspid valve (see Video 10-1). de-CMR (bottom) demonstrates central contrast uptake (yellow arrow), consistent with “tumor stalk” associated vascularity typical of cardiac myxoma.
Noncontrast imaging can also be useful to assess additional tissue characteristics. Myxomas may contain focal calcification, which can be well demonstrated on noncontrast CT as regions of markedly increased signal attenuation (ie, bright, Hounsfield Unit [HU] density > 130). The gelatinous, myxoid nature of the periphery of the myxoma results in high signal on T2-weighted CMR, and a similar mechanism underlies the frequently observed high signal of myxomas on cine steady-state free precession (SSFP) imaging. Hemorrhage may also be present, which can be demonstrated on T2-weighted (noncontrast) CMR as an area of diminished signal in an otherwise high-signal region.7
Lipoma
Epidemiology
Lipomas constitute approximately 8% to 12% of primary benign cardiac tumors.8 These masses can occur at any age and do not demonstrate a particular gender predominance.
Clinical Features
Cardiac lipomas may be asymptomatic, or present with symptoms secondary to impaired cardiac hemodynamics (ie, dyspnea, fatigue). The latter phenomenon is attributable to tumor mass effect (ie, if the lipoma is large in size and/or obstructs valvular flow).
Etiology/Tissue Composition
Cardiac lipomas are composed of encapsulated mature adipose cells (similar to extracardiac lipoma) without associated calcification, hemorrhage, or tissue necrosis.
Imaging Findings
• Location: Lipomas can occur in all cardiac chambers. These tumors can be subendocardial, epicardial, or mid-myocardial in location.
• Morphology: Lipomas are often wide-based with potential for large size.
• Tissue characteristics: On CT, lipomas appear dark due to low signal attenuation of adipose tissue (ie, HU density typically ≤ –30). On CMR, lipomas appear bright on T1-weighted imaging (Figure 10-3A) due to short T1 of adipose tissue. Fat suppression methods can be useful to further support this diagnosis, with lipomas (bright on T1) appearing dark on fat suppressed images. Cine-CMR can occasionally be useful to demonstrate fat due to “chemical shift” artifact (resulting from differences in the resonant frequencies of fat and water that produce signal dropout in affected pixels), which appears as peripheral thickening at the fat-water interface (Figure 10-3B). Of note, lipomas do not enhance on postcontrast imaging.
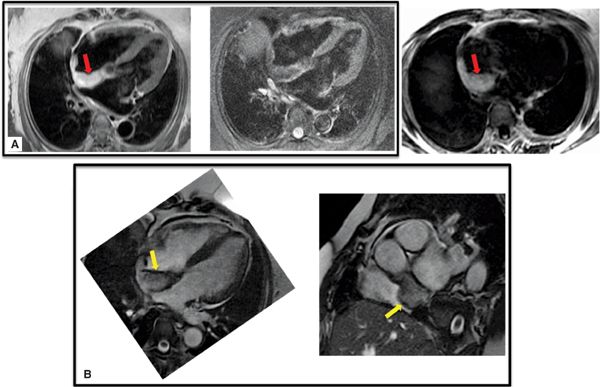
FIGURE 10-3 Lipoma. Right atrial lipoma with associated fat composition demonstrated on CMR tissue characterization imaging. (A) On T1-weighted spin-echo CMR (left), fat appears bright (red arrow) and can be made to appear dark with use of fat suppression methods (middle). Note involvement of the fossa ovalis, which can be used to distinguish lipoma from lipomatous hypertrophy (see Figure 10-19). Other CMR techniques, such as water-suppressed SSFP (right), can also be used to demonstrate lipoma-associated fat (red arrow). (B) Corresponding cine-CMR images demonstrating linear region of decreased signal intensity (yellow arrow), consistent with “chemical shift” artifact.
Rhabdomyoma
Epidemiology
Rhabdomyomas are the most common pediatric cardiac neoplasm, constituting approximately 50% to 75% of such cases.9–12 These tumors do not manifest a particular gender predominance and are typically diagnosed in-utero (ie, via screening ultrasound) or within 1 year of age.1,13,14 Rhabdomyomas can be associated with tuberous sclerosis (TS)—a multisystem condition involved with benign neurologic tumors (giant cell astrocytomas, cortical tubers, subependymal nodules) that can also be associated with renal, dermatologic, ophthalmic, pulmonary, and cardiac abnormalities. A combination of symptoms may include seizures, developmental delay, dermatologic abnormalities, pulmonary and/or renal dysfunction. Tuberous sclerosis is typically associated with mutation of either of 2 genes, TSC1 and TSC2, which code for the proteins hamartin and tuberin respectively.15
Etiology/Tissue Composition
Cardiac rhabdomyomas are composed of atypical myocytes containing abundant glycogen with marked cytoplasmic vacuolization.
Clinical Features
Rhabdomyomas may be clinically silent and can regress spontaneously. Clinical features associated with rhabdomyomas are nonspecific and can include cardiac arrhythmias and heart failure-related symptoms attributable to tumor mass effect.
Imaging Findings
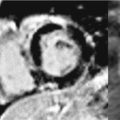
• Location: Rhabdomyomas typically arise in the LV or RV and often involve the interventricular septum (Figure 10-4). Rhabdomyomas are typically multifocal. However, in approximately 50% of sporadic cases (ie, occurring in the absence of TS), rhabdomyomas are single and not multiple. These rhabdomyomas often do not demonstrate spontaneous regression and may require surgery.16,17
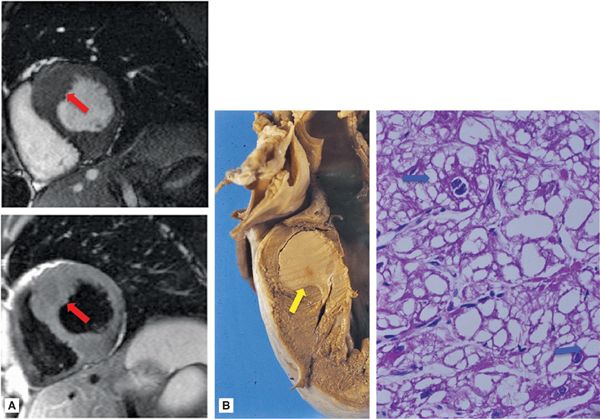
FIGURE 10-4 Rhabdomyoma. (A) CMR-evidenced septal hypertrophy in association with cardiac rhabdomyoma. Note: both cine (top) and spinecho (bottom) CMR demonstrate nonspecific septal hypertrophy (red arrows), without obvious evidence of a discrete intracardiac mass. (B) Gross picture of a cardiac rhabdomyoma (yellow arrow), which appears as a pale, circumscribed mass (~2 cm diameter). Microscopically, rhabdomyoma consists of clear cells. The typical cell of rhabdomyoma is the spider cell (blue arrows) that has vacuolated cytoplasm with strands of cytoplasm extending from the periphery of the cell to the nucleus (hematoxylin and eosin stain, magnification 40X).
• Morphology: Rhabdomyomas are often intramural and can produce localized distortions in endocardial contours. Cine-CMR and/or cine-CT can be useful to demonstrate focal regions with altered contractility.
• Tissue characteristics: Rhabdomyomas demonstrate minimal or no hyperenhancement on delayed-contrast CMR,2,8 with similar enhancement characteristics to be expected on contrast-enhanced CT (CE-CT). On noncontrast CMR, rhabdomyomas are typically iso- or minimally hypointense on T1–and slightly hyperintense on T2-weighted imaging in comparison to surrounding myocardium.2,13
Fibroma
Epidemiology
Fibromas are the second most common pediatric benign cardiac neoplasm.18 Patients typically present in early adolescence, although approximately one-third of patients present before 1 year of age. Fibromas rarely present in adulthood.
Clinical Features
Fibromas can present with cardiac arrhythmias and/or electrocardiographic abnormalities, which are likely attributable to myocardial (ie, septal) infiltration. Fibromas can also impede cardiac performance and result in heart failure symptoms.
Cardiac fibromas can also be associated with Gorlin syndrome—an autosomal dominant condition characterized by multisystem neoplasms involving skin (basal cell), bone (odontogenic keratocysts), and central nervous system (medulloblastoma).19
Etiology/Tissue Composition
Cardiac fibromas are composed of a homogeneous proliferation of fibroblasts interspersed with large amounts of collagen. Adjunctive calcification is common.
Imaging Findings
• Location: Fibromas typically occur within the LV and are most commonly adjacent to the interventricular septum. They are less commonly located adjacent to the LV lateral wall, within the RV, or within the LA, or RA.
• Morphology: Fibromas are typically mural (flat) in shape, with potential to disrupt adjacent endocardial or epicardial contours. Predilection for the interventricular septum can produce a morphologic appearance mimicking hypertrophic cardiomyopathy.
• Tissue characteristics: On delayed postcontrast MRI, fibromas demonstrate intense contrast uptake and appear hyperintense when inversion times are adjusted to null normal myocardium.20 Delayed postcontrast CT can also be used to identify vascular supply based on presence of contrast uptake.
Noncontrast imaging can also be useful for tissue characterization of fibromas. Noncontrast CT can be useful to demonstrate calcification, which appears as a region of markedly increased signal attenuation (ie, bright, HU density > 200). On noncontrast CMR, T1-weighted imaging typically demonstrates fibromas to be isointense, whereas they are hypointense (compared to normal myocardium) on T2-weighted imaging.21, 22 It should be noted that reports from pediatric series indicate that fibromas may not demonstrate hypointensity on T2-weighted imaging,2 possibly due to their more cellular content in younger patients.
Paraganglioma (Cardiac Pheochromocytoma)
Epidemiology
Cardiac paragangliomas are extremely rare.23 These tumors do not manifest a gender predominance and typically present in adulthood. While most are sporadic, case reports have associated paragangliomas with syndromic endocrinopathies (ie, multiple endocrine neoplasia type IIA)24.
Clinical Features
Systemic symptoms of cardiac paragangliomas result from catecholamine excess and may include palpitations, diaphoresis, flushing, or headache. Hypertension and tachycardia are typically present.
Etiology/Tissue Composition
Cardiac paragangliomas are composed of cardiac paraganglial (chromaffin) cells. Adjunctive histological features include hemorrhage and necrosis. Calcification is rare.
Imaging Findings
• Location: Paragangliomas most often occur in the LA, although they can originate at any site that contains normal cardiac ganglia (ie, atria, atrioventricular [AV] sulcus, coronary artery and/or great vessel origins)25.
• Morphology: These neoplasms are typically intramyocardial and without distinct shape.
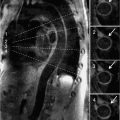
• Tissue characteristics: Paragangliomas typically demonstrate increased signal on noncontrast T2-weighted imaging (Figure 10-5), similar to paragangliomas in other body regions and often described as “lightbulb bright” using this approach.25 They characteristically demonstrate marked, intense contrast-enhancement on perfusion imaging, owing to their highly vascular nature. In addition, they may show heterogeneous signal intensity on postcontrast (CMR or CT) imaging, with variable decrements in contrast-enhancement attributable to localized regions of tissue necrosis.26
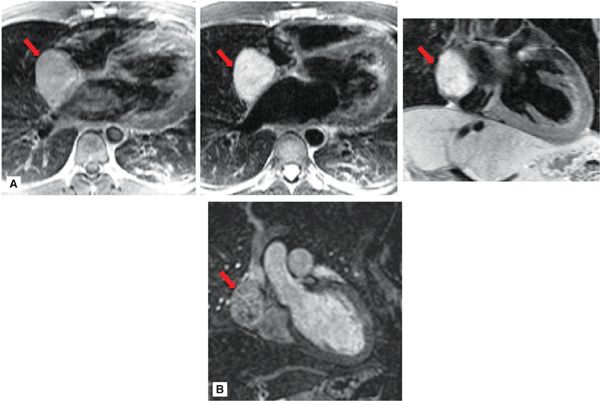
FIGURE 10-5 Paraganglioma. (A) Right atrial paraganglioma (red arrow), as assessed using T1- (left) and T2- (center, right) weighted CMR. Note iso-intense appearance on T1-weighted CMR, as compared to marked increased signal intensity on T2-weighted imaging. (B) Magnetic resonance angiography (coronal orientation) demonstrates heterogeneous contrast uptake within the paraganglioma, consistent with neoplasm-associated vascularity.
Hemangioma/Lymphangioma
Epidemiology
Both hemangiomas and lymphangiomas are unusual cardiac tumors, for which exact incidence is not established due to their rare nature. Hemangiomas present at variable ages ranging from infancy to late adulthood. Lymphangiomas typically occur in childhood or adolescence.
Clinical Features
Hemangiomas and lymphangiomas are typically asymptomatic and without associated clinical or syndromic features.
Etiology/Tissue Composition
These tumors are endothelial-lined, thin-walled masses containing either blood (hemangioma) or lymph (lymphangioma) that is isolated from the lymphatic system. Hemangiomas tend to demonstrate prominent endothelial channels with a variable proportion of fat. Lymphangiomas are often fluid-filled, containing endothelial-lined spaces that can become large and cystic in appearance. Both tumors contain prominent vascularity; neither typically involves calcification, hemorrhage, or necrosis.
Imaging Findings
• Location: Hemangiomas most often originate within the LV or RV (Figure 10-6A). Lymphangiomas have a predilection for the epicardial surface or pericardial space (Figure 10-6B).
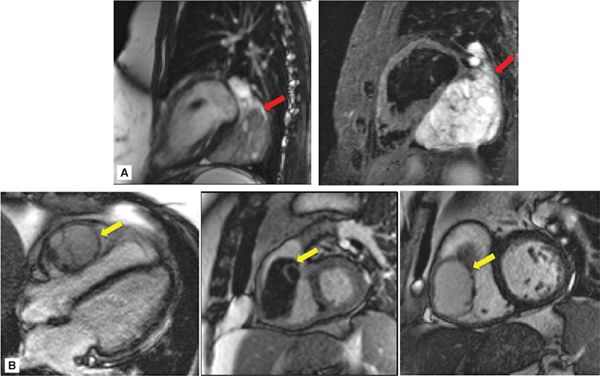
FIGURE 10-6 Hemangioma, Lymphangioma. (A) Hemangioma imaged using cine- (left) and T2-weighted spin-echo (right) CMR. This hemangioma (red arrow) was located adjacent to the inferior wall of the LV, consistent with ventricular predilection of these tumors. Note typical lobular appearance and high signal intensity on T2-weighted imaging, with the latter feature likely attributable to hemangioma-associated blood content. (B) Lymphangioma imaged using cine- (left, center) and DE- (right) CMR. In this case, the lymphangioma (yellow arrow) was right ventricular in location, lobular in shape, and without evidence of prominent vascular supply on DE-CMR.
• Morphology: Both tumors are typically lobular, broad-based, and mobile.
• Tissue characteristics: On postcontrast imaging, hemangiomas typically demonstrate heterogeneous enhancement in context of prominent vascular composition.8 The enhancement pattern of lymphangiomas has not been well described.
On noncontrast CMR, hemangiomas demonstrate high signal on T1-weighted images, likely due to the frequent presence of areas of interspersed fat. Lymphangiomas have low signal intensity on T1-weighted images due to their extensive cystic nature. Both are hyperintense on T2-weighted CMR,25,27 possibly due to high fluid content within lymphangiomas and nonclotted blood within hemangiomas.
CLINICAL MANAGEMENT
While clinical and imaging features are useful to narrow diagnostic considerations, such features can overlap and uncertainty can persist regarding patient-specific diagnoses. Distinction between benign and malignant neoplasms is an additional concern that may require direct pathological evaluation. Regardless of diagnostic etiology, many tumors hold the potential to impede cardiac function due to mass effect. In the context of these general considerations, surgical resection is often undertaken for excision of presumed benign tumors. Following resection, surveillance imaging may be useful to monitor for recurrence. Additional condition-specific considerations are detailed as follows:
Myxoma
• Genetic testing should be considered for associated chromosomal mutations (Carney complex). If positive, familial imaging should also be considered to screen for myxoma.
• Surveillance imaging is especially important for genetically affected patients, in whom likelihood for myxoma recurrence is increased.
Fibroma
• Clinical screening for other manifestations of Gorlin syndrome should be considered (ie, dermatologic, orthopedic, neurologic neoplasms).
Rhabdomyomas
• These tumors can be clinically silent and spontaneously resolved. Patients with rhabdomyomas are typically followed clinically with adjuvant imaging to monitor change in tumor size. Surgery is often reserved for symptomatic patients (ie, heart failure, arrhythmia).10,17
Paraganglioma
• Resection is typically curative, but can be technically challenging as paragangliomas can be highly vascular and may involve coronary arteries.
MALIGNANT NEOPLASMS
Malignant neoplasms constitute approximately 25% of primary cardiac tumors.1,28 Whereas sarcomas represent the most common primary malignancy, secondary (ie, metastatic) malignancies are far more common, with incidence rates over 10 times that of primary cardiac tumors.29 From an imaging standpoint, a common feature of neoplasms concerns vascular supply. Vascularity can be demonstrated as contrast-enhancement on tissue characterization imaging. This feature can be useful to distinguish neoplasms from avascular masses such as a thrombus.3 As an adjunctive approach, morphologic imaging can be helpful to assess tumor extent/invasion, with CT particularly well suited to assess involvement of neighboring extracardiac (ie, lung, bone, lymphatic) structures. The following section details key diagnostic features of malignant cardiac neoplasms.
DIFFERENTIAL DIAGNOSIS
Angiosarcoma
Epidemiology
Sarcomas are the most common primary cardiac malignancy, and nearly half are classified as angiosarcoma.1,30 Angioasarcomas manifest a slight male predominance and most commonly present in middleage.31 These tumors are highly aggressive tumors and often involve extracardiac metastases.
Etiology/Tissue Composition
Angiosarcomas are composed of endothelial and mesenchymal cells. On histopathology, they typically contain vascular channels lined by pleomorphic and atypical cells. Focal hemorrhage is commonly present.
Clinical Features
Angiosarcomas can present with a broad array of clinical symptoms. These often relate to RA or pericardial involvement and can include dyspnea, ascites, and/or lower extremity edema. Superior vena caval (SVC) flow obstruction can occur, resulting in venous distention/edema involving the upper extremities, chest, neck, and face (“superior vena caval syndrome”).
Imaging Findings
• Location: Angiosarcomas typically occur in the right atrium. Localized extension into LA or pericardium can occur, with the latter resulting in hemorrhagic pericardial effusion.
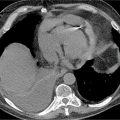
• Morphology: Two particular patterns can occur: (1) Angiosarcomas typically appear as bulky intracavitary masses (typically right atrial) that invade adjacent myocardium, chamber cavities, and/or pericardium (Figure 10-7); (2) less commonly, angiosarcomas can present with primary pericardial involvement, resulting in isolated pericardial thickening and/or pericardial effusion.
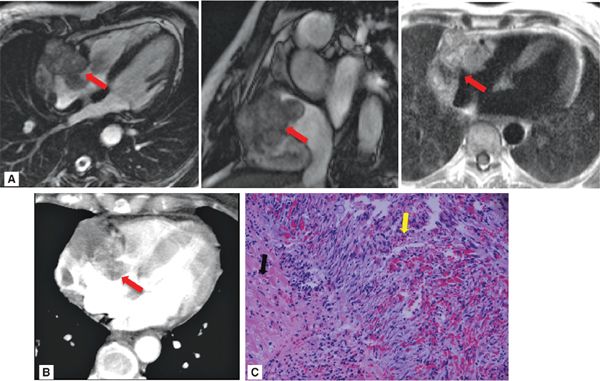
FIGURE 10-7 Angiosarcoma, as assessed by CMR and cardiac CT. (A) Cine- (left, center) and T1-weighted spin-echo (right) CMR demonstrate a large, irregularly contoured mass (red arrow) that demonstrates 2 typical features of angiosarcoma—RA location and pericardial involvement. (B) Contrast-enhanced CT demonstrates similar morphologic appearance as well as diffuse contrast uptake, consistent with prominent vascular supply. (C) Histopathology evidenced angiosarcoma (hematoxylin and eosin stain, magnification 20X). Note myocardium (black arrow), as well as angiosarcoma consisting of atypical spindle cells (yellow arrow) with areas of hemorrhage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree