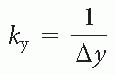Cardiac MRI
Introduction
Cardiac MRI is arguably the most difficult MRI examination to perform. Cardiac MRI must overcome not just respiratory motion, but also cardiac motion that cannot be suspended for the image. Additionally, there are a variety of nomenclature approaches to the pulse sequences, some of which are fairly unique to cardiac imaging while others are used in other organ systems all contributing to difficulty in understanding cardiac MRI. These different nomenclature systems are superimposed on the option of static versus cine imaging, and functional or physiologic imaging which all combine to challenge not just the patient and MRI technologist, but the MR physicist and radiologist.
Motion Effects and Compensation
Motion is a perpetual challenge to high-quality images in cardiac MRI. Body imagers are very knowledgeable of respiratory motion in abdominal imaging, but cardiac MRI has the additional challenge of cardiac motion. Respiratory and cardiac gating techniques are used frequently in cardiac MRI to compensate for motion. Gating techniques use an electrical impulse based on a physiologic marker (e.g., R wave from an electrocardiographic [ECG] or diaphragm position indicator) to accept, reject, or reorder data in k-space contributing to an image. Gating techniques fall into two broad categories: (i) prospective and (ii) retrospective. Prospective gating or EKG- or plethysmographic-triggering uses the impulse and based on previous preset or calculated parameters determines prospectively how k-space will be filled prior to signal acquisition. On the other hand, retrospective gating runs the pulse sequence and collects the signal regardless of the electrical or pressure impulse marker, and then either “on the fly” or after the signals have all been obtained uses certain parameters to either accept or reject signals for inclusion into k-space and subsequent Fourier transformation.
Respiratory Motion. Respiratory motion can be compensated by either breath-hold imaging or respiratory gating. The average individual can breath-hold for 15 to 25 sec, while individuals with cardiopulmonary disease will be able to breath-hold for shorter periods, which limits this technique. Alternatively, respiratory gating techniques track the motion of the diaphragm either indirectly by using a bellows device around the chest/abdomen or directly through a navigatorecho pulse. Both techniques track the depth and direction of the respiration and then either eliminate or minimize k-space acquisition when the position of the diaphragm falls outside of prescribed limits.
A bellows device can utilize either (i) respiratory gating (triggering) or (ii) respiratory compensation (respiratory-ordered phase encoding [ROPE] or centrally ordered phase encoding [COPE]) of k-space. Respiratory gating can be either prospective or retrospective. Gating will accept signals into k-space if the bellows detects the diaphragm position at or near a predetermined position in the respiratory cycle (usually
end-expiration). However, respiratory gating utilizes only about 20% of the respiratory cycle since most of the time the diaphragm position will lie outside the ideal position. Respiratory compensation (ROPE and COPE) improves efficiency by reordering the sequence of k-space filling based on the fact that the outer portions of k-space are less sensitive to changes due to motion than the central portion. ROPE gradually varies the phase-encode steps along the entirety of the respiratory cycle, while COPE acquires the lower phase-ordered steps (central k-space) around the longest respiratory cycle dwell time (usually end-expiration) with ever-increasing higher phase steps the further away from endexpiration.
end-expiration). However, respiratory gating utilizes only about 20% of the respiratory cycle since most of the time the diaphragm position will lie outside the ideal position. Respiratory compensation (ROPE and COPE) improves efficiency by reordering the sequence of k-space filling based on the fact that the outer portions of k-space are less sensitive to changes due to motion than the central portion. ROPE gradually varies the phase-encode steps along the entirety of the respiratory cycle, while COPE acquires the lower phase-ordered steps (central k-space) around the longest respiratory cycle dwell time (usually end-expiration) with ever-increasing higher phase steps the further away from endexpiration.
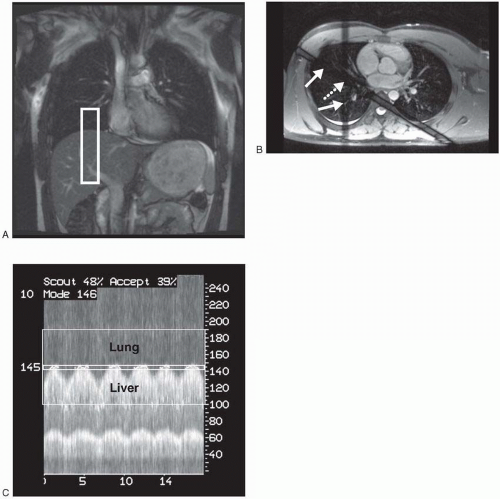 Figure 28-1. Navigator-echo gating. A: Coronal True FISP scout demonstrates column of excited tissue (rectangle) that the navigator-echo pulse will use on the right hemidiaphragm. B: Axial True FISP showing path of the intersecting RF pulses (arrows) generating the navigator-echo column at the intersection (dashed arrow). C: Tracing of navigator echo data showing the lung/liver interface and movement of the diaphragm. Time along x-axis and distance in millimeters along y-axis. Narrow window at “145” demonstrates acceptance window. |
The newest respiratory gating method is navigator–echo gating. This technique does not require the bellows, but instead uses either a single spiral radio frequency (RF) pulse or two intersecting RF pulses, usually on the right diaphragm, to track movement. Navigator-echo gating data then usually prospectively triggers the acquisition of kspace if the object tracked is within a certain prescribed window of movement (usually 3 to 5 mm) (Fig. 28-1). Newer variants of navigator gating
include a phase-reordered sequence that triggers the filling of the center of k-space when the diaphragm is within the ideal range while contributing to the outer portions of k-space when the diaphragm is outside the ideal range. This adaptation fills k-space during more of the respiratory cycle, resulting in a shorter scan time similar to respiratory compensation techniques. Lastly, navigator information can be used prospectively to correct for motion by adjusting the slice position if the diaphragm is detected outside the preferred window. This technique is known as slice-tracking or motion-correction.
include a phase-reordered sequence that triggers the filling of the center of k-space when the diaphragm is within the ideal range while contributing to the outer portions of k-space when the diaphragm is outside the ideal range. This adaptation fills k-space during more of the respiratory cycle, resulting in a shorter scan time similar to respiratory compensation techniques. Lastly, navigator information can be used prospectively to correct for motion by adjusting the slice position if the diaphragm is detected outside the preferred window. This technique is known as slice-tracking or motion-correction.
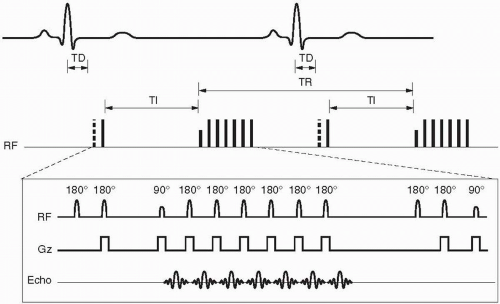
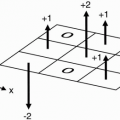
Cardiac Motion. Cardiac motion is complex with various contributions from longitudinal shortening (long-axis), radial contraction (shortaxis), and rotational motion. Although cardiac motion can be compensated for by using a pulse oximeter device or plethysmograph peripheral pulse monitor, ECG gating is more precise and usually yields a superior result. ECG gating allows the signal to be acquired in the same phase (e.g., systole and mid-diastole) of the cardiac cycle, resulting in less cardiac motion. However, there are additional complexities of the cardiac cycle with resultant R-R interval variability due to (i) normal beat-to-beat variability, (ii) premature contractions, and (iii) changes due to respiration especially breath-hold. Prior to scanning, the patient’s cardiac tracing is monitored and certain parameters are calculated based on the patient’s R-R interval range. The R-R interval range and frequency of premature contractions vary between patients and, if significant enough, can negatively impact image quality. ECG gating approaches these limitations and variables in two fundamental ways: (i) prospective and (ii) retrospective gating (Figs. 28-2 and 28-3). Additionally, for highly variable R-R interval patients, an arrhythmia reject window can be used in both gating techniques that further prevents filling k-space if R waves fall too far outside expected parameters. The arrhythmia reject window length may be either symmetric or asymmetric around the expected R wave.
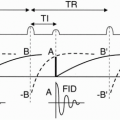
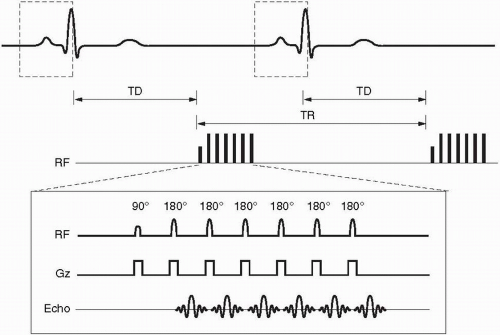 Figure 28-2. Prospective gating for a single slice/single phase (mid-diastole) fast spin-echo sequence with an echo train length = 6. Notice the long trigger delay (TD) between the R wave and the commencement of the pulse sequence with the initial 90° RF pulse. Dashed boxes around the QRS waves indicate the built-in arrhythmia reject window intrinsic to prospective gating. |
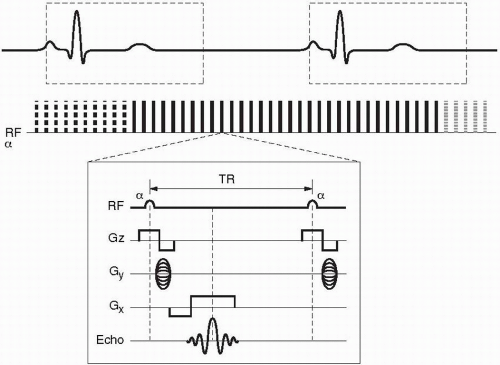 Figure 28-3. Retrospective gating for a single slice/multiphase cine sequence using a GRE pulse sequence. Notice the oversampling of the R-R interval of about 125% denoted by the different dashed or solid lines denoting a complete train of RF pulses contributing to a segment of k-space for the separate cardiac phases. There is no trigger delay as in prospective gating. Notice that the TR is much shorter in the GRE sequence than in the FSE sequence in Figure 28-2. Lastly, the dashed box represents an optional asymmetric arrhythmia rejection window (− 10% to + 50% expected R wave) whereupon an R wave detected outside of this box would result in rejection of the prior R-R interval’s signals. |
Prospective gating uses R wave detection with a variable trigger delay and then begins collecting k-space. The k-space is then filled over a certain prescribed percentage of the average R-R interval (usually 80% to 90% for cine imaging) whereupon no k-space is filled during the last 10% to 20% of the R-R interval. This results in a constant number of lines of k-space that are filled per R-R interval (Fig. 28-2). The last 10% to 20% of the R-R interval that does not fill k-space is designed in arrhythmia reject window that prevents acquisition of k-space during an early R wave due to beat-to-beat variability. However, this may or may not exclude premature contractions, and this can then be solved by superimposing a broader arrhythmia rejection window. Prospective gating is used in both static and cine imaging.
Retrospective gating, on the other hand, does not have any periods within the cardiac cycle where k-space is not being filled. This technique over samples the R-R interval usually at 125% and then retrospectively goes back and based on the detected R waves determines which line of k-space corresponds with each specific cardiac phase (Fig. 28-3). This technique results in a variable number of k-space lines filled per R-R interval since the R-R interval has beat-to-beat variability and sometimes premature contractions. After the collection of signals, the computer then uses a weighted interpolation technique and determines the phase of the cardiac cycle that each signal belongs to. This technique is most commonly used in cine sequences such as gradient-recalled echo (GRE), true fast imaging with steady-state precession (True FISP, Siemens), FIESTA (fast imaging employing steady-state acquisition, General Electric), or b-FFE (balanced fast field echo, Philips) and phase contrast imaging.
If arrhythmias are too frequent, then ECG triggering can become impossible, and one can salvage the study by taking off the cardiac gating and increasing the NEX to 4. The idea behind this is that systole is typically a small
percentage of most patients’ cardiac cycles, and thus by increasing the NEX, it will average out the smaller percentage of the signal acquired during systole with its greater motion. If the patient’s heart rate is fast, especially over 100, then this technique will be limited as well. The drawback is that scan time will increase and breath-hold techniques will be impossible, so a respiratory-gated technique must be used. Lastly, a single-shot technique (discussed later) can be used.
percentage of most patients’ cardiac cycles, and thus by increasing the NEX, it will average out the smaller percentage of the signal acquired during systole with its greater motion. If the patient’s heart rate is fast, especially over 100, then this technique will be limited as well. The drawback is that scan time will increase and breath-hold techniques will be impossible, so a respiratory-gated technique must be used. Lastly, a single-shot technique (discussed later) can be used.
Blood Motion. Blood movement within the heart and great vessels is pulsatile with complex flow characteristics, resulting in motion-induced intravoxel dephasing from differing velocities within a voxel contributing to signal loss. Valvular stenosis and regurgitation, vessel stenosis, and cardiac shunts can all have marked effects on the blood flow velocities, direction, and subsequent dephasing and signal loss. Flow compensation or gradient moment nulling can help mitigate these problems, but at a small sacrifice of a higher minimum time to echo (TE).
Faster Imaging. The faster the imaging sequence, the less chance for any type of motion-related effects. GRE or True FISP imaging with very short TRs or half-Fourier acquired single-shot turbo spin-echo (HASTE) sequences all acquire k-space in a shorter period of time versus fast spin-echo (FSE) sequences. A motion-reducing option for GRE or True FISP sequences is to acquire the k-space in a singleshot mode where the entire image’s k-space is acquired within a single R-R interval. HASTE sequences are by nature a single-shot sequence.
Additionally, parallel imaging can be used with any pulse sequence (see Chapter 24) and commonly reduces the time of acquisition by two to fourfold or increases spatial resolution two to four times without a time penalty. The major drawback to parallel imaging is decreased signal-to-noise ratio (SNR), so parallel imaging works best with sequences that have high SNR such as True FISP or postgadolinium imaging.
Motion in Cardiac Imaging and Solutions
Gross patient movement: instruct patient to lie still, mild sedation
Respiratory movement: breath-hold techniques, respiratory gating, navigator-echo gating
Cardiac motion: ECG gating, pulse oximeter gating, increase NEX, single-shot technique
Blood motion: flow compensation/gradient moment nulling, pulse sequences insensitive to dephasing, for example, True FISP
Parallel imaging: two to fourfold decrease in acquisition time however decreased SNR
General k-space Filling Strategies
Segmented versus Single Shot. Early in cardiac MRI, only one line of k-space was filled for a single image during a single R-R interval. This was a very inefficient way of filling k-space and resulted in long scan times. Subsequently, all cardiac imaging fills k-space in a segmented fashion that fills more than one line of k-space for a single image during a single R-R interval. One can conceptualize k-space “segmented” into separate areas based on different R-R interval contributions of k-space. For example, if there were 160 phase-encode steps and eight lines of k-space were filled for a single image during each R-R interval, then there would be 160/8 = 20 segments of k-space. The eight lines of k-space filled per R-R interval is termed views per segment (VPS). If all k-space is filled in a single R-R interval, then this is equivalent to a single segment, and it is referred to as a single shot. It should be noted that a “single shot” outside of cardiac MRI usually refers to a pulse sequence where a single RF pulse tips all the required longitudinal magnetization into the transverse plane for k-space to be completely filled for a single image such as in a HASTE or single-shot EPI sequence.
Static Imaging
All of the previously described pulse sequences in this book have been or are used to produce static cardiac images. These images are usually divided into two categories: (i) bright blood and (ii) dark blood. Unfortunately, the appearance of blood as either bright or dark is a consequence of the specific pulse sequence and not a particular sequence one can select on
the scanner. Blood signal has dark T1 and bright T2 signal intrinsically. Knowledge of the intrinsic signal of blood combined with superimposed time-of-flight effects (time-of-flight [TOF] loss or flow-related enhancement [FRE]) in a particular pulse sequence determines whether blood is bright or dark.
the scanner. Blood signal has dark T1 and bright T2 signal intrinsically. Knowledge of the intrinsic signal of blood combined with superimposed time-of-flight effects (time-of-flight [TOF] loss or flow-related enhancement [FRE]) in a particular pulse sequence determines whether blood is bright or dark.
Fast Spin Echo and HASTE. Spin-echo imaging has generally been abandoned due to excessive scan times, and it is now replaced by either FSE or HASTE that provides good anatomic detail, intrinsic dark-blood signal due to TOF loss, and appropriate T1 or T2 weighting based on TR and TE. FSE with ECG gating and either breath-hold or navigator-echo gating result in good image quality; however, the drawback is lengthy scan times. HASTE sequences have shorter scan times and are usually obtained in a single R-R interval; however, the SNR will be less due to 1/2NEX signal averages.
Although FSE and HASTE sequences are usually dark blood, some areas of slow blood flow from physiologic, pathologic, or in plane flow can result in minimal expected TOF loss and unexpected bright-blood signal confounding interpretation. This bright signal can be minimized by using a double-inversion recovery (DIR) sequence. This sequence uses a nonslice-selective 180° RF pulse followed immediately by a slice selective 180° RF pulse and an appropriate T1 that is influenced by the R-R interval (T1 usually 600 msec) that nulls the signal of inflowing blood, resulting in a more robust black blood effect (Figs. 28-4 and 28-5). Due to some intrinsic changes in the position of the slice from the initial 180° RF pulses and the actual filling of k-space about 600 msec later, the slice-selective 180° RF pulse is usually twice the nominal thickness of the slice to ensure that all the myocardium is reinverted. Additionally, to allow full recovery of the longitudinal magnetization, k-space is usually filled every other R-R interval unless the patient is bradycardic.
Fast Spin-Echo Gating. Static imaging including FSE uses prospective gating. Optimal gating with minimal motion artifact is achieved when
each image is acquired in the same cardiac phase. Static images are either acquired as a single slice/single phase (usually mid-diastole) or a multislice/single phase where multiple slices are acquired in an interleaved fashion. The single image/single phase technique fills k-space during mid-diastole and results in less motion since it minimizes changes of cardiac phase due to beat-tobeat variability; however, usually only one slice or at most two slices can be acquired during a single breath-hold (Fig. 28-2). Multislice/single phase is more efficient, but has more motion effects due to beat-to-beat variability and misregistration between slices due to the changing position of the heart at different phases of the cardiac cycle.
each image is acquired in the same cardiac phase. Static images are either acquired as a single slice/single phase (usually mid-diastole) or a multislice/single phase where multiple slices are acquired in an interleaved fashion. The single image/single phase technique fills k-space during mid-diastole and results in less motion since it minimizes changes of cardiac phase due to beat-tobeat variability; however, usually only one slice or at most two slices can be acquired during a single breath-hold (Fig. 28-2). Multislice/single phase is more efficient, but has more motion effects due to beat-to-beat variability and misregistration between slices due to the changing position of the heart at different phases of the cardiac cycle.
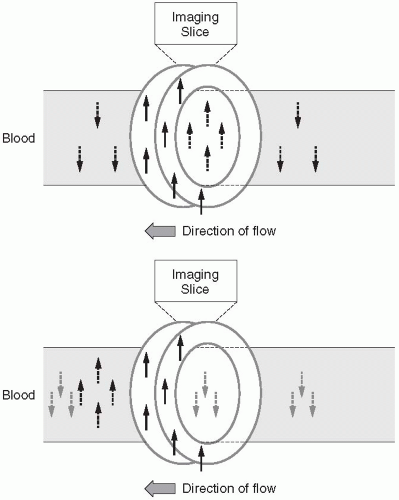 Figure 28-5. A:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|