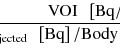Fig. 19.1
Typical coronary anatomy. The LAD, LCx, and RCA are the three main coronary arteries that perfuse the LV myocardium. The colored polar map is an example image of relative tracer uptake (% of maximum) in the LV
While cardiac SPECT is the workhorse of nuclear cardiology with an estimated 20,000 procedures per day in the USA alone being performed, cardiac PET is firmly established for state-of-the-art research applications and is exponentially expanding into clinical use. With the expected increases in SPECT radiopharmaceuticals costs, it is expected that the clinical use of cardiac PET will continue to grow in the coming decade [2]. Other imaging modalities (i.e., MRI, x-ray CT, and ultrasound) for cardiac applications are inherently different in their ability to image anatomy and tissue characterization, but less so for imaging molecular function, and therefore complement PET, hence the continued interest in hybrid imaging solutions such as PET-CT and PET-MR in cardiology.
19.2 Instrumentation
While most PET instrumentation has been designed with oncology application in mind, several adaptations have been crucial to enable cardiac PET. In the early 2000s, recognizing the increasing prevalence of obese patients, PET design shifted toward larger gantries with >60 cm diameter openings and beds capable of supporting upward of 200 kg patients. Faster, multislice diagnostic CT scanners became available as part of hybrid systems enabling coronary CT angiography and PET in a single imaging session – however, the routine clinical application remains rare.
ECG gating was introduced as a means to bin data into several (e.g., 8–24) phases of the cardiac cycle. Each coincidence event is placed in a bin corresponding to its cardiac contractile phase, and each bin is reconstructed individually producing a cine image of the beating heart. By viewing a single cardiac phase, a motion-free image is produced, offering greater spatial resolution. The greater the number of bins however, the fewer counts are available in each bin resulting in noisier images. In research applications, visualization of the mid-to-end diastolic (e.g., 70–80 % of the R–R interval) phase has been utilized as a relatively simple method to maximize image spatial resolution as the heart is nearly dilated (highest feature separation) and least moving (reduced motion blur).
The adoption of 3D PET acquisition highly benefited cardiac applications due to the two- to fivefold increase in camera sensitivity overall and as much as an order of magnitude increase in the central planes, which enables improved image quality especially with ECG-gated imaging [3]. Dynamic imaging with short imaging time frames also benefited from the improved count statistics of 3D PET. In static imaging, the increased camera sensitivity could be leveraged for shorter imaging times, radiation dose reductions, and/or reduced image noise. The addition of iterative statistical reconstruction and time-of-flight technologies has complemented 3D PET acquisition to further improve the effective sensitivity of cardiac PET.
With the exponential increase in data storage capacity, list-mode data acquisition has become a clinical reality and is offered by all vendors. In cardiac PET list-mode acquisitions were instrumental in enabling simultaneous acquisition of static, gated, and dynamic image series, primarily benefiting myocardial perfusion imaging (MPI) tests which is the most widely used application of cardiac PET [4]. A routine MPI exam consists of static and ECG-gated imaging of the tracer uptake for evaluating perfusion and cardiac function, respectively. An increasing number of centers also acquire a dynamic image sequence from the start of tracer administration to the end of initial distribution which is used to quantify myocardial blood flow (MBF), requiring early start of the image acquisition to measure the first-pass transit of tracer through the heart.
With the recent introduction of PET-MR hybrid scanners, new cardiac applications are being explored. One clinically relevant application of these hybrid systems is simultaneous PET and MR acquisition to reduce patient body and organ motion [5]. The MR images can be used to detect and characterize the motion, which is then incorporated into the PET image reconstruction to produce motion-free images. Furthermore, integration of PET acquisition with sophisticated cardiac MR protocols promises simultaneous acquisition of physiologic function from PET and high-quality anatomical and tissue characterization information from MR [6].
On the small animal imaging front, cameras have evolved to become extremely sensitive using longer bores and tightly packed detectors, enabling the use of lower tracer activities, even in the context of mice and rat imaging, keeping PET at the forefront of molecular imaging. Meanwhile, with continued improvements in imaging hardware and reconstruction algorithm, submillimeter imaging resolution is possible, but remains limited by the range of positrons leaving the atomic nucleus before combining with a free electron to produce the annihilation photons for PET imaging.
19.3 Cardiac PET Tracers
Cardiac PET applications are entirely dependent on the range of available molecules that can be imaged in vivo and therefore remains an active research field [7]. A unique advantage of PET over other noninvasive imaging modalities is the ability to image and quantify tracer concentrations in miniscule amounts due to the specific signal of the tracer and the very high sensitivity of PET instrumentation [8]. The molecular amount of radiolabeled molecule required for routine imaging is therefore in the pico- to femtomolar range, which is crucial to achieving trace quantities that do not invoke a physiologic response from the patient, organs, or cells [9, 10]. This property of PET is especially advantageous when probing mechanisms with limited binding capacity such as receptor signaling. Table 19.1 lists some of the more commonly used cardiac PET tracers, their characteristics, and their applications.
Table 19.1
Common cardiac tracers and their indicators
Tracer | Radioactive half-life (min) | Mean positron range in water (mm) | Production | Indications | Comments |
|---|---|---|---|---|---|
82Rb-rubidium chloride | 1.27 | 2.60 | Generator (82Sr) eluate | Myocardial perfusion and blood flow quantification | Reasonable uptake-to-flow relationship Requires direct injection system Suitable for rapid serial imaging |
13N-ammonia | 10.0 | 0.57 | Cyclotron – purified | Myocardial perfusion and blood flow quantification | Excellent uptake-to-flow relationship |
15O-water | 2.05 | 1.02 | Cyclotron – synthesized | Myocardial blood flow quantification | Requires direct injection from bedside synthesis unit Ideal uptake-to-flow relationship MPI not possible |
18F-flurpiridaz | 109.8 | 0.23 | Cyclotron – synthesized | Myocardial perfusion and blood flow quantification | Excellent uptake-to-flow relationship |
62Cu-pyruvaldehyde bis(N-methyl-thiosemicarbazone) (PTSM) | 9.67 | 4.39 | Generator (62Zn) eluate | Myocardial perfusion and blood flow quantification | Short parent isotope half-life has limited application |
18F-fluorodeoxyglucose (FDG) | 109.8 | 0.23 | Cyclotron – synthesized | Myocardial viability Inflammation (e.g., cardiac sarcoidosis, vulnerable plaque, device infection) Glucose uptake | Nonspecific cell target – explored for many biomarkers |
11C-glucose | 20.3 | 0.42 | Cyclotron – synthesized | Myocardial viability Glucose metabolism | Radiolabeled metabolites complicate quantification |
18F-fluorodeoxymannose (FDM) | 109.8 | 0.23 | Cyclotron – synthesized | Macrophage activity Glucose metabolism | Early studies for inflammation and oncology applications |
11C-acetate | 20.3 | 0.42 | Cyclotron – synthesized | Oxygen consumption myocardial perfusion and blood flow quantification | Indirect oxidative metabolism |
18F-fluorothia-6-heptadecanoic acid (FTHA) | 109.8 | 0.23 | Cyclotron – synthesized | Fatty acid uptake | |
16-[18F] fluoro-4-thia-palmitate (FTP) | 109.8 | 0.23 | Cyclotron – synthesized | Fatty acid uptake and metabolism | Hypoxia-dependent tissue uptake |
Trans-9(RS)-18F-fluoro-3,4(RS,RS)-methyleneheptadecanoic acid (FCPHA) | 109.8 | 0.23 | Cyclotron – synthesized | Fatty acid uptake | High cardiac tissue uptake and retention |
11C-palmitate | 20.3 | 0.42 | Cyclotron – synthesized | Fatty acid metabolism | Difficult kinetics due to metabolites |
11C-lactate | 20.3 | 0.42 | Cyclotron – synthesized | Lactate metabolism | |
15O2 | 2.05 | 1.02 | Cyclotron – purified | Oxygen consumption | |
11C-carbon monoxide | 20.3 | 0.42 | Cyclotron – synthesized | Blood volume imaging | |
15O-carbon monoxide | 2.05 | 1.02 | Cyclotron | Blood volume imaging | Requires direct inhalation from bedside synthesis unit Often used in conjunction with 15O-water perfusion scans |
11C-hydroxyephedrine (HED) | 20.3 | 0.42 | Cyclotron – synthesized | Sympathetic innervation |
19.4 Perfusion
19.4.1 Myocardial Perfusion Imaging
Myocardial perfusion imaging (MPI) with SPECT is widely used to diagnose epicardial coronary artery disease (CAD), to prognosticate for risk stratification, and to guide therapy [11]. PET has been shown to be cost-effective compared to alternative imaging modalities [12]. Patients are imaged at rest and following either exercise or pharmacologic stress. The reconstructed images are evaluated in conjunction to evaluate rest perfusion, stress perfusion, stress-rest reversibility (ischemia), and stress-rest flow reserve (the capacity to increase perfusion). MPI with PET has been shown to have improved diagnostic accuracy [13] and prognostic value [14, 15], largely due to improved image quality and robust attenuation correction. A recent meta-analysis of the current literature concluded that 82Rb-PET was superior to 99mTc-SPECT for detection of obstructive CAD (as defined by invasive coronary angiography) with 90 % vs. 85 % sensitivity and 88 % vs. 85 % specificity [16]. Consequently the use of PET to guide CAD patient management has been demonstrated to result in up to 50 % fewer invasive procedures, 30 % cost savings, and favorable outcomes when compared to SPECT [17]. However, due to the higher cost of PET, it has not been widely applied for MPI. The increasing availability of lower-cost PET cameras, the adoption of generator-produced 82Rb in high-throughput clinics, and the increasing costs of 99mTc isotope for SPECT tracers are fuelling the growth of the cardiac PET MPI market. Currently, 82Rb, 13N-ammonia, and 15O-water are approved for myocardial perfusion indications in some jurisdictions, with new tracers in various stages of regulatory review.
19.4.2 Stress Protocols
In general, exercise stress (e.g., treadmill) is preferred clinically, unless contraindicated, as it most accurately simulates real-life exertion and patient symptoms [18]. However, due to the short half-life of PET tracers, pharmacologic stress is routinely used for MPI studies. A variety of pharmacologic agents are available for inducing hyperemic flow during imaging, as listed in Table 19.2. Pharmacologic stress PET is advantageous for imaging at peak stress, as opposed to post-stress imaging which is performed with treadmill exercise and SPECT imaging. Some agents are designed to stimulate the sympathetic nervous system both directly and indirectly, and others induce vasodilation of coronary vessels, resulting in increased cardiac output to maintain arterial blood pressure. Since caffeine is a competing vasodilation agonist, absence of caffeine intake for >12 h is recommended to achieve maximum response between rest and stress [19].
Table 19.2
Commonly use cardiac stress agents in clinical MPI and MBF quantification
Agent | Response | Mechanism | Dose | Half-life | Remarks | Specific contraindications |
|---|---|---|---|---|---|---|
Adenosine | Vasodilation | Coronary A2a smooth vessel agonist | 0.14 mg/kg/min | 30 s | Caffeine has similar structure and also is an agonist of A1,A2B,A3 receptors | Asthma >Second-degree AV block or sinus node dysfunction (without artificial pacemaker) Bradycardia SBP <90 mmHg Unstable acute MI or coronary syndrome |
Dipyridamole (Persantine) | Vasodilation | Inhibit adenosine reuptake and increases the intramyocardial concentration | 0.14 mg/kg/min | 40 min | Aminophylline used as antidote | |
Regadenoson | Vasodilation | Coronary smooth vessel agonist on A2a | 0.4 mg | 2–3 min | No need to stop β-blockers. No need for IV line (bolus injection) | >Second-degree AV block or sinus node dysfunction (without artificial pacemaker) SBP <90 mmHg |
Dobutamine | Chronotropic and inotropic | Strong β1, moderate β2, and mild α1 adrenergic receptor agonist | 10–40 μg/kg/min | Very short | Could be combined with atropine | Recent MI Unstable angina Severe LV outflow obstruction History or risk of LV tachycardia Uncontrolled hypertension Aortic dissection or aneurysm Use of β-blockers |
Combined use of different stress agents can be used to evaluate individual contributing mechanisms to myocardial flow reserve. A common example is the use of the cold pressor test (CPT), in which a limb is submerged into ice-cold water, invoking a systemic vascular response which is dependent on endothelial function through adrenergic stimulation. The endothelium-mediated vasodilation represents a fraction of the total maximal flow obtained from stress agents above [20]. CPT and rest flow measurement have been used to demonstrate endothelial dysfunction in smokers compared to nonsmokers and that endothelial function may be rapidly restored with smoking cessation [21]. CPT can be unpleasant and difficult to endure, and alternative agonists of the sympathetic system such as induced hypercapnia [22] or salbutamol [23] are under investigation.
19.4.2.1 MPI Interpretation
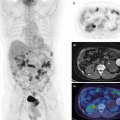
Regional myocardial perfusion is evaluated visually by comparing tracer uptake in different regions of the myocardium against a reference region of maximum uptake, which is assumed to be normally perfused. Regions with homogeneous uptake at rest and stress are interpreted as normal. Regions with relatively reduced tracer uptake at stress that normalize at rest are interpreted as ischemic, while regions with relatively reduced uptake at both rest and stress are interpreted as myocardial scar [18]. The rest and stress images are reoriented to a standard orientation that can be viewed as a series of short-axis (SA) slices transecting the LV from apex to base as demonstrated in the top rows of Fig. 19.2. Orthogonal slices may be displayed to generate horizontal and vertical long axis (HLA and VLA, respectively) views of the heart (middle and bottom row on images). A more concise presentation of the LV is using polar maps (right bottom) which sample the mid myocardium contour. The apex is presented at the center of the polar map with the base at the radial extreme; the septum and anterior, lateral, and posterior walls are on the left, top, right, and bottom, respectively, as illustrated in Fig. 19.1.


Fig. 19.2
Example of myocardial perfusion (MPI) report for a 54-year-old male patient using 82Rb PET. A large uptake deficit in the septal, anterior, posterior, and apical regions at stress that is resolved at rest as indicated by the reversibility polar map
LV polar maps can be segmented into regions which are each scored individually to generate a semiquantitative score of defect severity. Most commonly a 17-segment model is used with scores ranging from 0 to 4 corresponding to normal perfusion, mild reduction, moderate reduction, severe reduction, and an absence of tracer uptake [24]. Summation of the segment scores produces summed stress and rest scores (SSS and SRS) which are global metrics of defect extent and severity. The summed difference score (SDS) is a global indicator of reversible ischemia. SSS >3 is a commonly used threshold for the presence of disease with SRS >3 and SDS >1 indicating the presence of nonreversible scar. A limitation of these scores is the dependence on segment alignment and visual assessment (including overriding of automatically generated scores) which reduces their reproducibility [25]. The total perfusion deficit (TPD) is a quantitative alternative which does not rely on polar map segmentation [26] and is therefore increasing in popularity.
Relative uptake image interpretation, however, is susceptible to misdiagnosing or underdiagnosing CAD in patient with uniform reduction in perfusion such as in balanced disease in multiple coronary vessels and in distributed disease of the microvasculature (e.g., associated with diabetes mellitus). Furthermore, since the most commonly used PET MPI tracer (82Rb) is extracted nonlinearly with blood flow, mild perfusion deficits may be difficult to detect. Absolute quantification of myocardial blood flow helps to address these limitations of relative-scale MPI.
19.4.2.2 Myocardial Blood Flow (MBF) and Flow Reserve (MFR) Quantification
With dynamic PET imaging, absolute myocardial blood flow (MBF) can be quantified at peak stress and at rest in units of mL/min per g of tissue (mL/min/g) (Fig. 19.4). The ratio of the two is termed the myocardial flow reserve (MFR) and indicates the capacity to increase blood flow to the heart in order to meet demand with exercise [4]. Flow difference (delta), the difference between MBF at stress and rest, may also be used to describe reserve in absolute terms (mL/min/g). MFR has been shown to increase sensitivity for detection of balanced multivessel disease, where the relative perfusion is normalized to the area of highest perfusion which is itself underperfused and therefore would appear normal on standard relative MPI. MBF and MFR in this region would be low, thereby unmasking ischemia [27–29]. In addition MFR has been shown to detect diffuse disease of the microvasculature as well as endothelial dysfunction which are markers of incipient epicardial CAD [30]. As a result, MFR has demonstrated prognostic value incremental to relative MPI for prediction of both mortality and major adverse coronary events [29]. Normal human populations have been used to define thresholds for abnormal MBF and MFR values. Typically MFR values >2.5 are associated with normal myocardial perfusion and blood flow. Conversely, an MFR <2 is associated with CAD and/or microvascular disease [31–33].
Stress MBF and MFR have been proposed as potential differentiators between functionally significant and benign coronary lesions. MBF at maximum hyperemia decreases progressively once the stenosis is greater than 40–50 % of the vessel diameter, while resting MBF remains normal until there is a diameter stenosis of 80–90 % [34], leading to the use of invasive assessment of fractional flow reserve (FFR) to guide invasive interventions with better specificity and outcomes [35]. MBF can be increased with revascularization [34], but in diabetics with focal narrowing, revascularization leads to only mildly improved blood flow [36]. These findings confirm that MFR is determined by a combination of epicardial vessel flow, functional microvasculature, and cell integrity [34]. While it has been demonstrated that spatial patterns of MPI along with other information can result in better patient outcomes when used to guide therapy [14], the same has not yet been demonstrated for absolute MBF and MFR quantification.
Quantification of MBF requires dynamic imaging from the time of tracer administration until distribution is achieved (typically 5–10 min). The acquisition is binned into predefined time frames ranging from several seconds long in the early time frames, where rapid dynamics are occurring, to a few minutes in length in the late time frames, where the dynamics are slow and count rates are relatively low. The dynamic image sequence is then processed using specialized software that models the tracer kinetics in the myocardial tissue and the arterial blood that perfuses it. In most cases myocardial regions of interest (ROI) are automatically, semi-automatically, or manually defined using an uptake image in which the myocardium is clearly visible. Likewise an arterial ROI is defined in the left ventricle, left atrium, or aorta. The ROIs are then used to sample the dynamic sequence to measure time-activity curves (TAC) of the blood (input) and myocardium (output). A parameterized kinetic model is used to describe the relationship between the blood and myocardium TACs. The model parameters are fit to the measured TACs. Typically a two-compartment (blood and tissue) model is sufficient [37], of which the blood-to-myocardium uptake parameter (K1) is related to MBF [4, 38]. A tissue washout parameter (k2) may be associated with tissue integrity, viability, or perfusion, depending on the tracer. K1 values must be corrected for flow-dependent extraction as discussed in the MPI tracer section in order to estimate MBF. See Chap. 14.
Special considerations must be given to tracer administration with dynamic PET imaging especially with short-lived isotopes such as 82Rb. Early in the imaging process, the tracer activity is concentrated in a relatively small blood volume, but is then distributed throughout the entire body and undergoes radioactive decay. Thus, early time frames may experience count rates of 3–4 orders of magnitude greater than those at late time frames as demonstrated in Fig. 19.3.


Fig. 19.3
Blood and myocardial time-activity curves of a 82Rb stress scan with and without isotope decay correction. While for kinetic modeling decay correction is required, in practice the PET camera measures the activity without decay correction, which varies over several orders of magnitude during the scan due to tracer distribution and radioactive decay
MBF and MFR analysis of the same patient as in Figs. 19.2 and 19.4 is shown in Fig. 19.5. While the MPI interpretation suggests ischemia in much of the LV territory that is resolved at rest, MBF and MFR paint a more severe picture of abnormally low MBF at stress in almost the entire LV and uniformly low rest MBF as well. Abnormal MFR (<2) and delta (<1 mL/min/g) are indicated in a larger territory (58 % of the myocardium) than with MPI, suggesting involvement of more tissue than indicated by MPI. Finally, cardiac steal (lower flow at stress than at rest) is indicated in 26 % of the LV also demonstrating severe obstruction of the blood supply in the territory of LAD artery.



Fig. 19.4
Myocardial blood flow quantification using a dynamic 82Rb scan (same stress scan in Fig. 19.2). An uptake image is generated using the late time frames and is reoriented (top left) to generate standard SA, HLA, and VLA views (top center) on which LV myocardium contours are defined (red lines) along with blood pool regions (A, B, and C regions). 3D meshes (top right) are displayed corresponding to the uptake polar map (bottom center). A one-tissue compartment model can be fit to myocardial (blue) and blood (red) time-activity curves (bottom left) to generate K1 and extraction corrected flow polar maps (bottom right) in units of mL/min/g

Fig. 19.5
Flow reserve using relative MPI (left) and absolute MBF (right) for the same patient as in Figs. 19.2 and 19.4. Similar stress deficit patterns are observed with both MPI and MBF, with more contrast and broader extent using MBF. Rest flow is uniform in both cases indicating stress-induced ischemia. The reserve and delta polar maps tell a similar story; however, MBF indicates a much more severe deficit than MPI, as indicated by cardiac steal (lower stress than rest flows) in 26 % of the LV territories using the clinical classification rules
Using automated classification rules [32], the territories associated with the left anterior descending (LAD) and mid to distal left circumflex (LCx) and right coronary arteries (RCA) were suspected to have obstructive stenoses. These findings were confirmed using invasive angiography as shown in Fig. 19.6 which indicated 100 % obstruction of the mid-LAD artery, 90 % stenosis in the first diagonal, >70 % stenoses in the proximal LCx and its branches, and >80 % stenoses in the distal RCA branches.


Fig. 19.6
Angiography for the same case as in Figs. 19.2, 19.4, and 19.5, indicating severe 100 % obstruction of the mid-LAD artery, 90 % stenosis in the first diagonal, >70 % stenoses in the proximal LCx and its branches (left), and >80 % stenoses in the distal RCA branches (right). Location of stenoses are indicated by red arrows
In MPI imaging, patient, organ and respiratory motions is manifested as a blur in the direction of motion which can lead to artifactual uptake deficits in opposing cardiac walls [39]. In MBF quantification these same motions can further result in inconsistent ROI sampling and attenuation misalignment artifacts that propagate to inaccurate TACs and MBF measurement errors. Quality assurance at each processing step can often expose the presence to motion clewing the reader to potentially unreliable MBF estimates. Robust motion detection and correction algorithms are an active area of research, but are complicated by rapid changes of the tracer distribution in the early time frames of the dynamic image sequence.
The effects of cardiac motion on MBF quantification are assumed to be small due to partial volume correction and due to improved normal-to-defect wall contrast as a result of tracer extraction correction. However in MPI, cardiac motion can produce regionally varying partial volume effects that can mask defects. For example, a hypokinetic wall region with mild uptake deficit may appear as bright as a normally perfused region with preserved wall motion and partial volume loses. For this and other reasons, MPI images should be interpreted in conjunction with ECG-gated imaging.
19.4.3 ECG-Gated Imaging
Whenever possible, ventricular function with gated imaging should be interpreted as part of MPI, as wall motion and wall thickening can be used to differentiate between ischemia and residual attenuation artifacts in regions with reduced tracer uptake [13]. Hypoperfused regions associated with wall motion abnormalities often signify scarred, stunned, or hibernating myocardium. However, if wall motion is preserved, apparent perfusion defects may be artifacts (e.g., attenuation misregistration, motion). Areas of normal perfusion with hypokinesis may be markers of myocardial stunning after an acute ischemic insult [11] or markers of mild perfusion deficit that are masked by lower regional partial volume losses (associated with reduced motion) compared to normal reference regions.
All current PET scanners offer the possibility of ECG gating and list-mode acquisition and therefore are able to reconstruct cine-gated images, dynamic images, and static (ungated) uptake MPI from a single injection and acquisition. An important advantage of physiologic stressing and image acquisition at peak stress is that wall motion abnormalities can be observed at peak stress, making PET more sensitive than conventional SPECT practice to detect regional and global wall motion abnormalities, which may otherwise resolve with post-stress imaging [13]. For the example case in Figs. 19.4, 19.5, and 19.6, the gated stress image indicated hypokinesis of the septal wall and dyskinesis of the apex, consistent with the findings of severe ischemia and steal from the MPI and MBF interpretations (Fig. 19.7).


Fig. 19.7
Cardiac wall motion at peak stress for the same patient example as in Figs. 19.2, 19.4, 19.5, and 19.6. The white mesh indicates LV cavity contours at end diastole and the color surface at end systole. The surface color indicates the extent of cardiac wall motion of the respective territory. The septal wall is hypokinetic, and the apex is dyskinetic corresponding to the MPI and MBF findings
19.4.4 MPI Tracers
As Table 19.2 demonstrates, several PET perfusion tracers are available, of which 82Rb is the most widely used. 82Rb is generator produced, and therefore does not require an on-site cyclotron compared to 13N-ammonia, 11C-acetate, or 15O-water. Flurpiridaz, as with other 18F-based MPI tracers, has a 2-h physical half-life and therefore may be distributed over large distances as is common with FDG and thus may become a clinically relevant alternative in the future.
The short half-life of 82Rb implies low radiation dose to the patient [40] and staff, but can contribute to low count statistics in late uptake images. Furthermore 82Rb has a relatively high positron range which can degrade the image spatial resolution slightly. 82Rb is extracted from the blood into perfused tissue both through diffusion and active ion transport, as it is a potassium analogue. Consequently, tracer extraction from the blood into the tissue space is limited (Table 19.3) and decreases nonlinearly with flow as demonstrated in Fig. 19.8. This effect must be accounted for in MBF quantification using an extraction correction function [4, 33, 41–43]. Thus K1 estimation error is amplified during conversion to MBF, especially at high stress flow values. As demonstrated in Fig. 19.8, several extraction correction functions have been reported in the literature; their differences likely demonstrate the use of different imaging equipment, analysis software, experimental approaches, and patient cohorts, and therefore the extraction function can also be interpreted as a calibration function of K1 to MBF (Fig. 19.8-right). Nevertheless, equivalent results have been demonstrated across commercially available software packages when using the one-tissue compartment model and the Lortie extraction function [44, 45]. The move to 3D PET imaging with higher count-rate capabilities is expected to improve the precision and accuracy of MBF using 82Rb in particular.
Table 19.3
Commonly use PET MPI and MBF tracer properties
Tracer | First-pass extraction | Image spatial resolution | Extracardiac interference | Injected activity with 3D PET (approximate) | Effective dose | Comments |
|---|---|---|---|---|---|---|
82Rb-rubidium chloride | 40–100 % dependent on flow | 8–12 mm | Stomach wall
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|