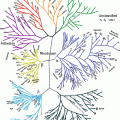
Fig. 6.1
The structure of the Common Technical Document (CTD) pyramid. Module 1 is region specific and modules 2, 3, 4 and 5 are intended to be common for all regions. The topics within the red circle are the components of the abridged application (From http://www.ich.org/products/ctd.html)
Many radiopharmaceuticals are manufactured directly to the final drug product; i.e. the drug substance or active pharmaceutical ingredient (API) is not isolated before formulation. This has some consequences for the CTD. Section “6.3.2. P Drug Product (Name, Dosage Form)” will be essentially the same as Sect. “6.3.2. S Drug Substance (Name, Manufacturer)” [24]. Copy and paste these sections; do not use different wording as this may confuse the assessor at the regulatory body. There will more about the language for applications in “scientific writing” section.
For each marketed product, there must be a Summary of Product Characteristics (SPC or SmPC) which presents information about the product, including dosimetry and instructions for its use. The EMA is developing standard SPCs for generic products [26].
6.4 Scientific Writing
My words fly up, my thoughts remain below; Words without thoughts never to heaven go (Claudius in Shakespeare’s “Hamlet”, Act III, scene iii).
Any application submitted to a regulatory body or competent authority must be very stringent and sober in both structure and language. It is, unquestionably, not a scientific paper to pass a peer review, but a description of drug manufacturing and quality control. Not all assessors are experts on radiopharmaceuticals and will thus react to any ambiguous or unclear wording.
Example: How Not to Write
Radionuclidic impurities
Radioactive oxygen-14 and nitrogen-13 may form as a result of minor side reactions in the cyclotron target: 14N(p,n)14O and 16O(p,α)13N. N-13 and O-14 are both positron-emitting radionuclides. The presence of O-14 in the drug substance may be ruled out as a significant contaminant because it has an ultrashort half-life of O-14 (T 1/2 = 70.6 s); thus in the course of synthesis ca 25 min, it will decay to 4.7 × 10-7 of its original amount. N-13 can appear in the form of gaseous 13N-labelled oxides (e.g. [13N]NO, [13N]NO2, etc.). The presence of 13N would alter the measured half-life of the drug substance and would be seen as a radiochemical impurity during chromatographic analyses. This has not yet been observed. It is worth noting that 11C in its process from starting material [11C]CO2 to the final product undergoes several chemical transformation steps with intermediate purifications. Therefore the probability for unwanted N-13 and O-14 species to survive through the same pathway as C-11 and end up in the final formulation is expected to be very low.
This is lengthy and confusing. Stating “not yet been observed” implies that it may be observed. Similarly, “the probability for unwanted” implies that there is a probability.
Example: How to Write
Radionuclidic impurities
Radioactive nitrogen-13 may form as a result of a minor side reaction in the cyclotron target: 16O(p,α)13N. N-13 can appear in the form of gaseous 13N-labelled oxides (e.g. [13N]NO, [13N]NO2, etc.). However, contamination of the final product with 13N is not possible because the chemistry of nitrogen oxides is different from that of the starting material, [11C]CO2, which undergoes multiple chemical transformation steps with intermediate purifications.
This is short and sweet. By saying “is not possible”, there is no doubt that it cannot be present. The language should be concise and formal, not verbose and/or sounding like a scientific investigation.
Example: “The product is purified by means of XXX and no impurities have been found” rather than “The product is purified by means of XXX, the possible impurity YYY which can potentially be produced by…..has so far not been detected”.
Example: “All batches passed the sterility test”, not “All batches passed the sterility test. However…”
Example: “The product is thus safe for human use”, not “The product is thus safe for human use, yet some investigations have found…”
Have enough data/information to make a clear statement. It is better to spend a few hours extra in the laboratory than spending a few months in discussions with the competent authority.
6.5 Good Manufacturing Practice (GMP)
6.5.1 Components of GMP
The goal of pharmaceutical GMP is to ensure a consistent high-quality product which is appropriate for its intended use and which meets the specifications of its marketing authorisation. Because standards are constantly changing, the term current good manufacturing practice, cGMP, is often used. The three main components of GMP are facilities and equipment, personnel and procedures and documentation, all carried out within a quality management system. European rules for GMP are described in Eudralex volume 4 [27] with specific reference to radiopharmaceuticals in annex 3 [28] and investigational medicinal products in annex 13 [29].
6.5.1.1 Facilities and Equipment
The design and construction of facilities must afford a clean environment. Surfaces must be impervious and able to withstand regular cleaning. Activities must be segregated with interlocking doors to prevent cross-contamination. There must be a cascade of filtered air pressure from the cleanest area to the exterior to minimise the ingress of particles. This is achieved by heating, ventilation and air conditioning system (HVAC) with high-efficiency particulate air (HEPA) filters. Similarly, there must be a regimen of gowning up to prevent staff from carrying contaminants into the clean areas. Materials are sanitised and then transferred in via hatches with interlocking doors. Aseptic processes must be carried out within an appropriate environment of filtered air. Generally, radiosynthesis can be carried out in Grade C, but sterile filtration and dispensing must be carried out in Grade A. These are generally achieved with a hot cell, pharmaceutical isolator or laminar airflow cabinet. All equipment must be kept in good working order with regular checks of performance and records of maintenance undertaken. Proper operation of the facility must be documented by routine environmental and microbiological monitoring. This includes a combination of measurements of air flows and air change rates, active air sampling and particle counting and routine microbiological monitoring including agar settle plates, contact plates and finger dab plates. All of this is covered by the site master file (SMF).
6.5.1.2 Personnel
Personnel must be adequately trained in any procedures they carry out, with a training record. The training record must indicate that the member of staff has read the SOP and has demonstrated competence with evidence of a certain number of monitored activities. The responsibilities and authorities of each grade of staff must be specified. Persons who perform aseptic procedures must undergo periodic revalidation of their competence. If production facilities are shared with a research institution, the research personnel must be adequately trained in GMP regulations, and the quality controller (see below) must review and approve the research activities to ensure that they do not pose any hazard to the manufacturing of radiopharmaceuticals.
There must be a person designated as production manager, sometimes called responsible person. This person is responsible for the routine operation of the facility to ensure that the radiopharmaceuticals are produced to GMP standards. This includes ensuring that there are sufficient numbers of suitably trained staff available on a daily basis. This person makes sure that all documentation is complete before products are passed on to the quality assurance department.
There must be another independent person designated as quality controller. This person takes ultimate responsibility for the quality of the radiopharmaceuticals produced, though the act of release may be delegated to a suitable trained person. In some situations the quality controller might also be a qualified person.
6.5.1.3 Procedures and Documentation
All Standard Operating Procedures (SOPs) must be written down, approved and reviewed at regular intervals. There must be contemporaneous manufacturing records which document the adherence to SOPs and record the identities and quantities of all materials used and any in-process checks, all of which must be initialled or signed by the operator and checker. Quality assurance results must include evidence that the analytical equipment had been properly calibrated. There must be a Validation Master Plan (VMP).
One of the underpinning features of GMP is the quality of starting materials. Precursors must be obtained from approved vendors. There must be a process for auditing or inspecting vendors. It may be possible for a single such audit to be performed on behalf of a number of PET centres in the same country. All chemicals which remain in the final formulation must be obtained from certified pharmaceutical suppliers. In particular, active pharmaceutical ingredients (API) must be obtained from a certified GMP source.
6.5.1.4 Quality Management System (QMS)
In recent years, inspectors have placed increasing emphasis on the quality management system [30]. Indeed, this chapter was revised in 2013. Quality management is a wide-ranging concept, encompassing all matters which individually or collectively influence the quality of a product. The components of a QMS include the following. Product and process knowledge is managed throughout all life cycle stages. Managerial responsibilities are clearly specified. Arrangements are made for the manufacture, supply and use of the correct starting and packaging materials, the selection and monitoring of suppliers and verifying that each delivery is from the approved supply chain. Processes are in place to assure the management of outsourced activities. The results of product and process monitoring are taken into account in batch release, in the investigation of deviations and with a view of taking preventive action to avoid potential deviations occurring in the future. All necessary controls on intermediate products and any other in-process controls and validations are carried out. Where the true root cause (s) of the issue cannot be determined, consideration should be given to identifying the most likely root cause (s) and to addressing those in order to assess which might be responsible. Where human error is suspected or identified as the cause, this should be justified having taken care to ensure that process, procedural or system-based errors or problems have not been overlooked. Appropriate corrective actions and/or preventative actions (CAPAs) should be identified and taken in response to investigations in a timely manner. The effectiveness of such actions should be monitored and assessed, in line with quality risk management principles. There is a process for self-inspection and/or quality audit, which regularly appraises the effectiveness and applicability of the pharmaceutical QMS.
6.5.2 Challenges for PET
There are two separate challenges for the application of GMP to the manufacture of PET radiopharmaceuticals. The first is dictated by the short half-lives of most radionuclides used in PET, e.g. 18F, 109 min; 11C, 20 min; 13N, 10 min; and 15O, 2 min. There is no time for all quality parameters to be assessed before release for clinical use. Following risk assessment it must be specified which parameters can be determined retrospectively. This would include sterility testing and determination of levels of residual solvents. If at all possible, endotoxin testing, as a surrogate of sterility testing, should be performed before release, while sterility testing is performed post release. Endotoxins are heat-stable toxins released from the cell wall of gram-negative bacteria; thus, the absence of endotoxins suggests that the product does not contain bacteria. Before a new product is introduced, there must be a number of validation runs with full analysis to establish the quality of the product and its reproducibility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree