CHAPTER 67 Cardiac Tumors
BENIGN CARDIAC TUMORS
Myxoma
Definition
Cardiac myxomas are benign neoplasms of endocardial origin, most commonly located in the atria.
Prevalence
With an incidence of 0.03%, cardiac myxoma is the most common primary cardiac tumor.1 It represents approximately 50% of all benign cardiac tumors.2 Myxomas have been reported in patients of every age, but most frequently patients present with myxomas between the third and sixth decades, with an average age of approximately 50 years.2,3 There is a higher prevalence in women.2
A rare autosomal dominant form of cardiac myxoma is termed the Carney complex. Associated features include pigmented skin lesions, cutaneous myxomas, primary pigmented nodular adrenocortical disease, mammary myxoid fibroadenomas, large cell calcifying Sertoli-cell tumors, pituitary adenomas, thyroid tumors, and melanotic schwannomas.4 Patients with the Carney complex present at an earlier age compared with patients with sporadic cases of cardiac myxoma, with an average age of 26 years.4 These patients are also more predisposed to develop multiple tumors, documented in 41%4 compared with 6% of patients with sporadic cardiac myxomas.5
Pathology
Cardiac myxomas arise from the endothelial surface by either a narrow or a broad-based pedicle and extend into the cardiac chamber (intracavitary growth) (Fig. 67-1). They range in size from 1 to 15 cm in diameter (average 5 to 6 cm).2 Approximately 75% originate in the left atrium, 15% to 20% originate in the right atrium, and 5% are biatrial.1 Myxomas rarely occur within the ventricular chamber or along the atrioventricular valves. Most are smoothly marginated, firm, and fibrotic (Fig. 67-2). The remaining one third to one half are gelatinous and friable with a villous or frondlike surface (Fig. 67-3).1 This latter type is the morphology typically found in the Carney complex and is more likely to produce embolization.6 Focal areas of hemorrhage may be evident on the surface of a myxoma.
Histologically, myxomas consist of inflammatory cells and myxoma cells in a myxoid matrix (Fig. 67-4). Myxoma cells are stellate multinucleate cells that form elongated cords and rings.6 They are of uncertain origin, but may arise from residual embryonal multipotential mesenchymal cells of the heart.2 Myxomas also characteristically contain cysts, hemorrhage, extramedullary hematopoiesis and, rarely, glandular elements.6 Calcification is common microscopically, and for unknown reasons is more prevalent in lesions on the right side of the heart.1
Manifestations of Disease
Clinical Presentation
Clinical presentation is extremely varied and depends on location, morphology, and size of the myxoma. The classic clinical triad is intracardiac obstruction, embolization, and constitutional symptoms.2 Approximately 20% of patients are asymptomatic.5 The most common symptoms of cardiac myxomas are secondary to obstruction.3 Obstruction from left atrial myxomas mimics mitral valve stenosis, causing dyspnea and orthopnea from pulmonary edema. Obstructive right atrial myxomas may produce peripheral edema and syncope. Ventricular myxomas may mimic aortic or pulmonic valve stenosis, causing syncope. Obstruction may be intermittent and positional with pedunculated tumors.3 Sudden cardiac death is rare, caused by temporary complete obstruction of the mitral or tricuspid valve.2 Additionally, the motion of pedunculated atrial tumors may cause incomplete closure of, or damage to, the atrioventricular valve apparatus.2
Emboli have been reported in 35% of left-sided and 10% of right-sided cardiac myxomas.3 Most emboli are systemic, originating from left-sided lesions or paradoxical emboli originating from right atrial myxomas. They may affect the cerebral, visceral, renal, peripheral, or coronary arteries. Clinically evident pulmonary emboli are infrequent, but have been reported in right-sided cardiac myxomas.2
Constitutional symptoms, including fever, fatigue, and weight loss, occur in approximately one third of patients.3 Other reported symptoms include arthralgias, myalgias, rashes, clubbing, cyanosis, and Raynaud phenomenon.2 Cardiac arrhythmias or palpitations occur in 20% of patients with myxomas.3 Less common presentations include chest pain, anemia, and sepsis.
Imaging Techniques and Findings
Radiography
Findings on chest radiograph vary with tumor location. Approximately 50% of left atrial myxomas produce findings suggestive of mitral valve obstruction, such as left atrial enlargement, prominence of the left atrial appendage, vascular redistribution, and pulmonary edema (Fig. 67-5).3 Cardiomegaly and pleural effusions are less frequent findings, but may occur with either right-sided or left-sided myxomas. Radiographically evident calcification is reported in 50% of right atrial myxomas, but is not seen in left atrial myxomas.3 Approximately one third of chest radiographs are normal.3
Ultrasonography
Transesophageal echocardiography may allow for better visualization of atrial tumors compared with the transthoracic approach (Fig. 67-6). Myxomas are pedunculated mobile masses on echocardiography, often apparently attached to the interatrial septum by a narrow stalk.7 Myxomas may be homogeneous or heterogeneous, with echogenic foci owing to calcification and hypoechoic areas from hemorrhage, necrosis, or cysts.7 Dynamic prolapse of the mass across the atrioventricular valve is often well visualized on echocardiography.
Computed Tomography
On contrast-enhanced CT, cardiac myxomas appear as intracavitary round or ovoid filling defects with smooth or lobulated contours.3 Most myxomas are hypodense to myocardium and unopacified blood (Fig. 67-7).3,5 Myxomas tend to enhance heterogeneously after administration of contrast medium (Fig. 67-8).3 CT may show a narrow base of attachment to the interatrial septum, although a pedicle is usually not as well seen as with echocardiography.7 Calcification may be evident, but typically only in right heart myxomas. Secondary complications of myxomas may be identified on CT, including pulmonary or visceral emboli and evolving infarction.3
Magnetic Resonance Imaging
On MRI, myxomas are usually isointense on T1-weighted sequences and high signal intensity on T2-weighted sequences because of their myxoid stroma composition (Fig. 67-9A and B).8 Myxomas are typically heterogeneous, likely reflective of varying components of hemorrhage, calcification, cysts, and myxoid or fibrous tissue.3 Loss of signal intensity occurs with gradient-recalled-echo (GRE) imaging possibly because of magnetic susceptibility from high iron content.3 Myxomas are usually hypointense to blood pool and hyperintense to myocardium on steady-state free precession (SSFP) sequences, although they may be isointense to blood and possibly missed on this sequence.9 Myxomas enhance with gadolinium, usually with a heterogeneous pattern on perfusion and delayed enhancement phases (Fig. 67-9C).10 MRI has been reported to be more accurate than CT in predicting the point of attachment to the wall, which may be best seen on cine images.3 Cine MRI also may show prolapse of the tumor across a cardiac valve (Fig. 67-10).10
Treatment Options
To prevent complications such as embolization or sudden cardiac death owing to valvular obstruction, the treatment of cardiac myxoma is prompt surgical resection. The stalk and zone of attachment are excised, including the full thickness of the interatrial septum for myxomas that arise in this location. Long-term prognosis is excellent. Recurrence occurs in less than 3% of nonfamilial cases and is considered related to incomplete resection.2 In patients with the Carney complex, recurrence is 20%, which may be due in part to multifocality of lesions.2,4
Papillary Fibroelastoma
Prevalence
Cardiac papillary fibroelastomas are the second most common primary cardiac tumor, representing approximately 10% of benign cardiac tumors.7 They are the most common tumor of the cardiac valves. Papillary fibroelastomas have been reported in all ages, but are most frequently found in the fourth to eighth decades of life, with a mean age of 60 years.11 In the largest case analysis, there was a slight male predominance of 55%.11 This lesion is usually solitary, and no familial cases have been described.
Pathology
Papillary fibroelastomas consist of multiple fronds attached to the endocardium by a short pedicle. When immersed in water or saline, they are described as having the appearance of a sea anemone (Fig. 67-11).1 The papillary fronds are avascular with an elastic fiber and collagen core surrounded by myxomatous matrix and endothelial cells.6 Dystrophic calcification has been reported, but is rare.11 Most lesions are approximately 1 cm in maximum diameter, although lesions up to 7 cm in size have been reported.11 More than 75% of papillary fibroelastomas are found on the cardiac valves, involving the aortic, mitral, tricuspid, and pulmonary valves in decreasing frequency.11 Aortic and pulmonary valve papillary fibroelastomas most commonly project into the vascular lumen, whereas papillary fibroelastomas on the atrioventricular valves usually project into the atria.11 They may also occur along the endocardial surfaces of the atria and ventricles or on the eustachian valve.
Manifestations of Disease
Clinical Presentation
Most papillary fibroelastomas are found incidentally during imaging, cardiac surgery, or autopsy.11 No longitudinal studies have been performed, and the natural history of these lesions is unknown. The most common clinical manifestation is embolism. Either the tumor itself or associated thrombus may embolize into the cerebral, visceral, renal, peripheral, coronary, or, less frequently, pulmonary arteries.11 Heart failure, arrhythmia, syncope, and sudden death are less common manifestations.
Imaging Techniques and Findings
Radiography
Because of their small size, papillary fibroelastomas would not be expected to produce any findings on chest radiography. If there is obstruction of the mitral valve, findings of pulmonary venous hypertension may be apparent. Calcification is rarely visible.11
Ultrasonography
Most papillary fibroelastomas are found incidentally during echocardiography. They typically appear as small, homogeneous, valvular masses that may be sessile or pedunculated.5 A stippled pattern may be seen near the edges, reflective of the papillary projections.11 Almost half of papillary fibroelastomas are mobile with evidence of flutter or prolapse.11,12
Computed Tomography
The few studies of the CT appearance of papillary fibroelastomas have used ECG gating. They describe a tiny, well-defined spherical mass attached to a valve leaflet (Fig. 67-12).13,14
Magnetic Resonance Imaging
Because of their small size, papillary fibroelastomas are infrequently visualized on MRI. Papillary fibroelastoma is typically a mobile, nodular mass with homogeneous intermediate signal intensity on T1-weighted sequences and intermediate or low signal intensity on T2-weighted sequences.14,15 It is best appreciated on cine MRI sequences as a small valvular mass with adjacent turbulent blood flow.10 It is characteristically hypointense to myocardium on GRE sequences.16 After administration of gadolinium, papillary fibroelastomas show delayed hyperenhancement possibly because of their fibroelastic tissue composition.13,17
Fibroma
Prevalence
Cardiac fibromas are the second most common tumor in childhood after rhabdomyoma. In contrast to rhabdomyomas, they are uniformly solitary.5 One third of patients present before 1 year of age, although 15% of cardiac fibromas are discovered in adolescence and adulthood.5 There is no sex predilection.18 Fibromas are associated with Gorlin syndrome (also known as basal cell nevus syndrome), an autosomal dominant syndrome of multiple basal cell carcinomas, odontogenic keratocysts, skeletal anomalies, and other neoplasms such as medulloblastoma.8
Pathology
Fibromas are mural-based whorled masses of white tissue (Fig. 67-13). Mean size is 5 cm (range 2 to 10 cm).5,7 They are not encapsulated and may have either circumscribed or infiltrating margins. Fibromas are typically located in the interventricular septum or left ventricular free wall.1,6 Less frequently, they are found in the right ventricle or atria. In newborns and infants, fibromas are highly cellular with numerous fibroblasts. With age, cellularity decreases, and the amount of collagen increases.6,19 Fibromas contain microscopic calcification in one third of cases; they are otherwise fairly homogeneous on histologic and gross examination.6
Manifestations of Disease
Clinical Presentation
Fibromas are asymptomatic in one third to one half of cases. Symptoms include heart failure, arrhythmia, chest pain, syncope, and sudden death.1,20 In one study of primary cardiac tumors that caused sudden cardiac death, fibroma was the second most common underlying cause (after endodermal heterotopia of the atrioventricular node).21
Imaging Techniques and Findings
Radiography
The most common abnormality on chest radiograph is cardiomegaly.22 A focal bulge of the cardiac contour can also be seen.19 Calcification overlying the cardiac silhouette is visualized radiographically in approximately one quarter of cases, and may appear dense or amorphous.5,20
Ultrasonography
Cardiac fibroma may appear as a discrete mass or a focal area of wall thickening mimicking focal hypertrophic cardiomyopathy on echocardiogram. Fibromas are echogenic and may appear heterogeneous.5
Computed Tomography
Fibromas are circumscribed or infiltrative mural-based masses on CT (Fig. 67-14A). They show soft tissue attenuation and often calcification.8 Enhancement may be either homogeneous or heterogeneous.5,19
Magnetic Resonance Imaging
On MRI, a cardiac fibroma appears as an intramural mass or focal myocardial thickening on T1-weighted sequences, where it is isointense or hypointense to myocardium (Fig. 67-14B).8,23 In contrast to other cardiac tumors, fibromas are characteristically hypointense on T2-weighted and SSFP sequences because of their fibrous tissue composition, which has low water content.8,23,24 The fibrous tissue is also hypovascular, causing little or no enhancement on perfusion imaging (Fig. 67-14C).24 On myocardial delayed enhancement MRI, fibromas typically show marked hyperenhancement, however, occasionally with central regions of hypointensity (see Fig. 67-14C).24 The delayed enhancement is currently attributed to the significant extracellular space for gadolinium accumulation.24
Treatment Options
In contrast to soft tissue fibromatosis, there is little evidence that cardiac fibromas may enlarge, and spontaneous regression has been reported.1 Treatment for symptomatic patients is complete surgical excision, although partial excision may be performed for more extensive tumors. Postsurgical recurrence is rare.5
Rhabdomyoma
Prevalence
Rhabdomyoma is the most common cardiac neoplasm in infants and children, and accounts for 50% to 75% of pediatric cardiac tumors.10 It occurs equally in boys and girls.10 Approximately 50% of patients with rhabdomyomas have tuberous sclerosis, an association that increases to 95% in fetuses and neonates with multiple cardiac tumors.25 Conversely, virtually all infants with tuberous sclerosis have cardiac rhabdomyomas, although this incidence decreases with age because of spontaneous regression.8 Rhabdomyoma is rarely associated with congenital heart diseases, such as Ebstein anomaly, tetralogy of Fallot, and hypoplastic left heart syndrome.22
Pathology
Rhabdomyomas usually consist of circumscribed mural-based nodules that average 1 to 3 cm in size; multiple nodules are present in 70% to 90% of cases.1 The left ventricle or interventricular septum is the most common location, followed by the right ventricle and atria in decreasing frequency.26 Rhabdomyomas protrude into the cardiac chambers in 50%,27 a characteristic observed more often in patients who do not have tuberous sclerosis.6 Histologically, the rhabdomyoma cell has a distinctive spider-like appearance with vacuolated cytoplasm and radiating myofibers surrounding a central nucleus. The cells stain with periodic acid–Schiff because of their high glycogen content.5
Manifestations of Disease
Clinical Presentation
Rhabdomyomas are most commonly diagnosed incidentally on routine second-trimester fetal ultrasound examinations.27 The most frequent presentation in utero is arrhythmia, including tachycardias and bradycardias.26 Additional fetal manifestations include hydrops and fetal death. Infants may be asymptomatic or present with arrhythmia, heart failure, or left ventricular outflow obstruction.26
Imaging Techniques and Findings
Computed Tomography
CT scan may show multiple intramural nodules that appear either hypodense or hyperdense to normal myocardium.28
Magnetic Resonance Imaging
Cardiac rhabdomyomas are intramural masses that are isointense on T1-weighted MRI sequences and hyperintense on T2-weighted sequences (Fig. 67-15).8 They may produce focal abnormalities of contractility.8 Rhabdomyomas enhance homogeneously and intensely with gadolinium.8,10 Numerous rhabdomyomas less than 1 mm in size, so-called rhabdomyomatosis, may produce diffuse myocardial thickening without a discrete mass.53
Lipoma
Prevalence
Lipomas reportedly represent 8% of primary cardiac tumors,29 although this may be an overestimate because many series do not differentiate lipomas from LHIS. They may manifest at any age, but patients are typically younger than patients with lipomatous hypertrophy.30 There is no gender prevalence.29
Pathology
Lipomas are circumscribed masses of homogeneous yellow fat that may be found within the myocardium, occasionally with extension into the pericardial space or cardiac chambers. The most common sites are the right atrium, left ventricle, and interatrial septum.29 Half are subendocardial, with the remaining half split between myocardial and subepicardial locations.29 Histologically, lipomas are encapsulated masses of mature fat cells, without the brown fat or hypertrophic myocytes found in lipomatous hypertrophy.
Manifestations of Disease
Clinical Presentation
Most cardiac lipomas are asymptomatic and are discovered during imaging or autopsy. Occasionally, they may cause obstruction or arrhythmias.8
Imaging Techniques and Findings
Ultrasonography
Lipomas are nonspecific homogeneous, immobile echogenic masses on echocardiography.5
Computed Tomography
CT and MRI have the advantage over echocardiography of being able to characterize the tissue type. Lipomas have homogeneous low attenuation on CT similar to subcutaneous or mediastinal fat, typically less than −50 Hounsfield units (Fig. 67-16). Thin strands of soft tissue attenuation septa may be present without any nodularity. No significant enhancement occurs with administration of contrast medium.
Magnetic Resonance Imaging
On MRI, lipomas are homogeneous, smoothly contoured masses. They show homogeneous fat signal intensity on all sequences, including decreased signal intensity on fat-suppressed sequences, and no enhancement with gadolinium.8,29 There may be thin septations, but no nodular components. Chemical shift artifact occurs on SSFP sequences at the interface between the lipoma and the myocardium or blood pool, resulting in a low signal intensity margin.29
Lipomatous Hypertrophy of the Interatrial Septum
Definition
LHIS is not a true neoplasm, but rather a benign diffuse thickening (defined as >2 cm) of the normal extension of epicardial fat within the interatrial groove.30
Prevalence
LHIS is significantly more common than true cardiac lipomas. In a prospective study of sequential chest CT scans, the incidence was 2.2%.31 The reported age range is 22 to 91 years, with most patients presenting with LHIS older than 60 years.30 Associations include age, obesity, pulmonary emphysema, and long-term parenteral nutrition.31 Some reports suggest a slight female predominance.30,31
Pathology
Lipomatous hypertrophy is a misnomer because the lesion actually represents hyperplasia rather than hypertrophy of the normal fat found within the interatrial septum. LHIS is a nonencapsulated, poorly circumscribed thickening of the fat of the interatrial groove that extends from the aortic root caudally to the coronary sinus; it is occasionally contiguous with mediastinal fat. LHIS spares the fossa ovalis, causing a bilobed appearance. In a prospective CT study, the thickness ranged from 2 to 6.2 cm (mean 3.2 cm).31 A combination of vacuolated brown fat cells, hypertrophic myocytes, and normal mature fat cells is seen on histologic examination.6
Manifestations of Disease
Clinical Presentation
Although most often an incidental finding, LHIS can be associated with supraventricular arrhythmias, sudden cardiac death, and heart failure.31

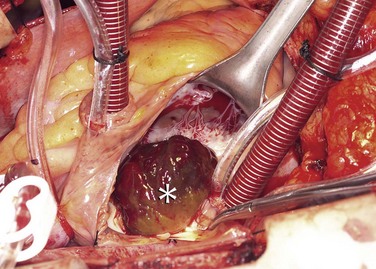
 FIGURE 67-1
FIGURE 67-1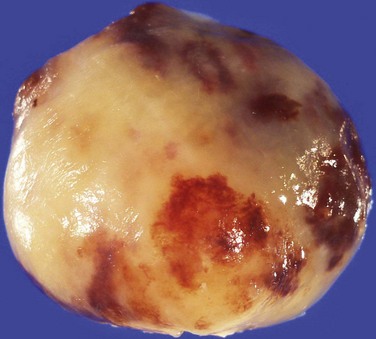
 FIGURE 67-2
FIGURE 67-2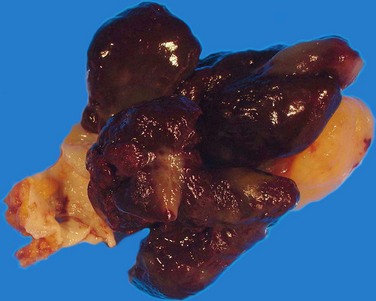
 FIGURE 67-3
FIGURE 67-3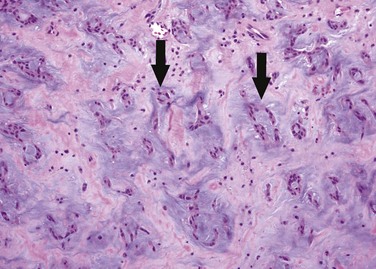
 FIGURE 67-4
FIGURE 67-4
 FIGURE 67-5
FIGURE 67-5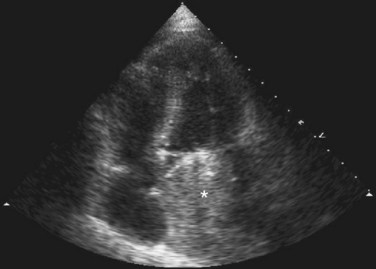
 FIGURE 67-6
FIGURE 67-6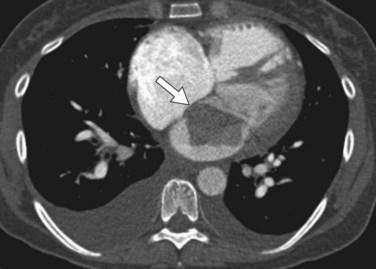
 FIGURE 67-7
FIGURE 67-7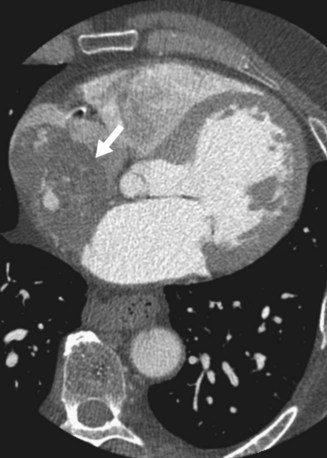
 FIGURE 67-8
FIGURE 67-8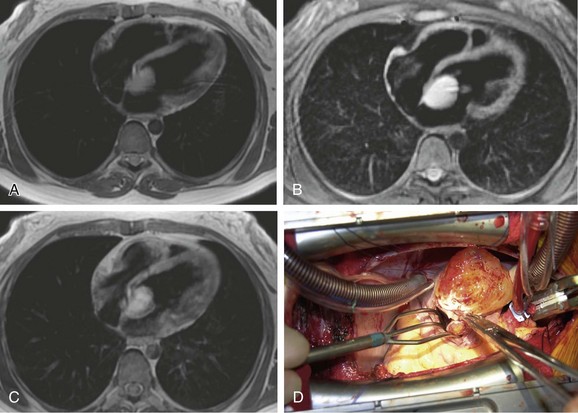
 FIGURE 67-9
FIGURE 67-9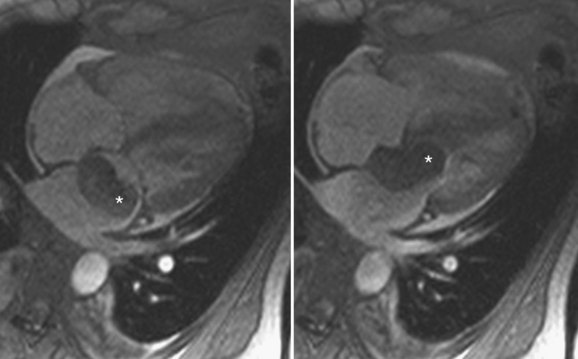
 FIGURE 67-10
FIGURE 67-10


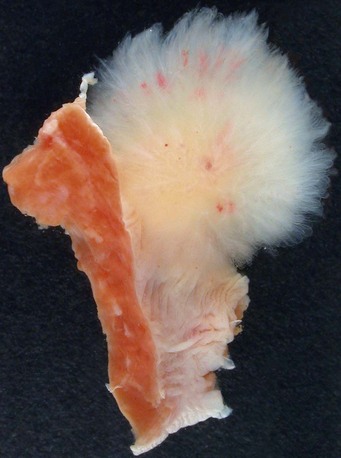
 FIGURE 67-11
FIGURE 67-11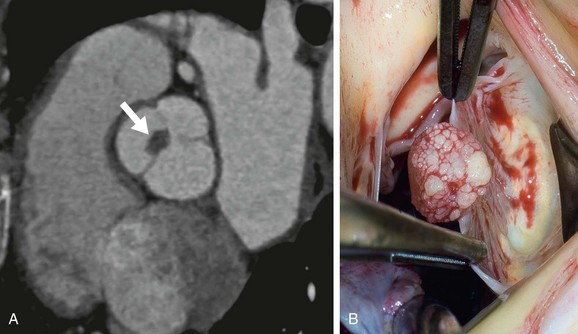
 FIGURE 67-12
FIGURE 67-12

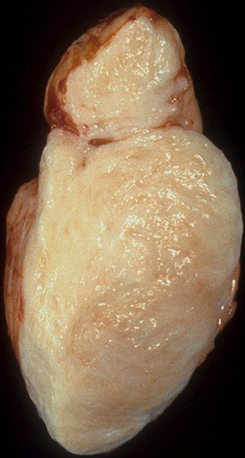
 FIGURE 67-13
FIGURE 67-13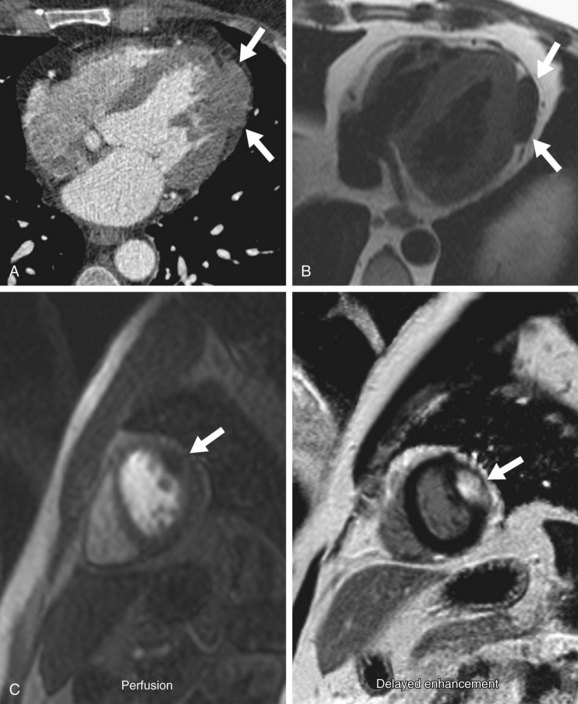
 FIGURE 67-14
FIGURE 67-14

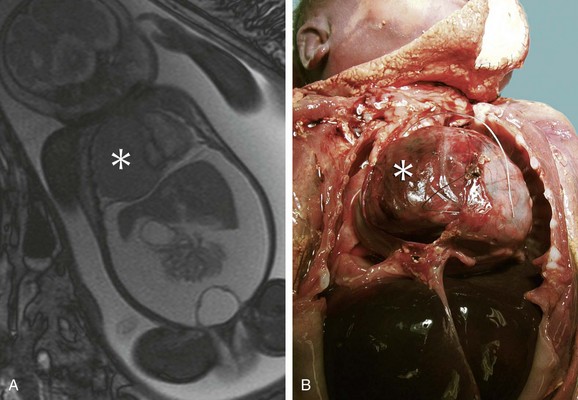
 FIGURE 67-15
FIGURE 67-15


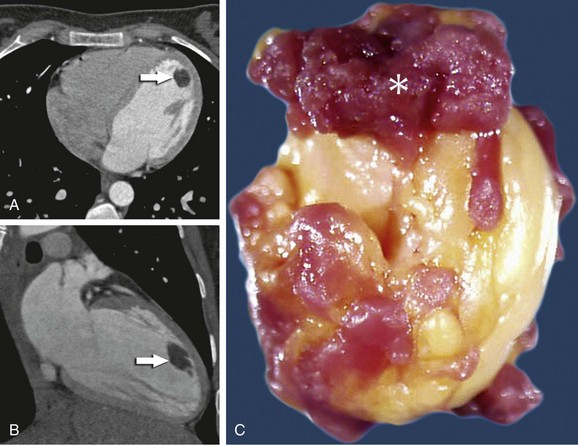
 FIGURE 67-16
FIGURE 67-16



