Chapter 32 Cardiovascular system
Equipment
Digital subtraction angiography (DSA) has been available for over 20 years. It is noteworthy that in the 2000 NCEPOD (National Confidential Enquiry into Perioperative Deaths)1 report on interventional vascular radiology it was stated that 8% of hospitals in the UK were still undertaking vascular work on barium screening systems. No recent, more up-to-date data are available, but it seems likely that this situation is now much improved, and certainly it should be considered unacceptable to be performing complex vascular imaging and intervention without access to DSA.
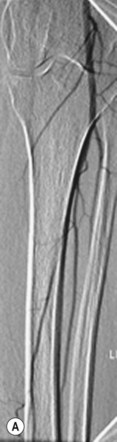
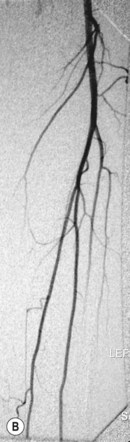
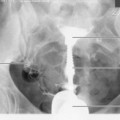
In general DSA is an excellent technique, but there can be problems with image quality, particularly due to patient movement. One method of dealing with this is to use a facility termed pixel shifting. This involves using the computer to move the mask and contrast images relative to one another such that they are properly aligned, thereby removing misregistration artefact (Fig. 32.1A,B). This method of image processing is most suited to movement in relatively simple anatomical structures such as a limb, and also in situations where movement has only been slight. More extreme movements can be very difficult, if not impossible, to correct by pixel shifting. This is because pixel shifting involves a simple translation of image data in two dimensions, whereas patient movement actually occurs in three dimensions, often involving a degree of rotation, rather than pure translation. Modern automated methods of pixel shifting can be of benefit where more extreme patient movement has occurred; however, there are still limitations to the technique, and attention to good patient positioning and explanation remains critical to obtaining good results.
Technique
The transfemoral approach
Complications of the transfemoral route are minimal during diagnostic arteriography; however, the recommended upper limit of complications for audit purposes is as high as 3%.2
The transbrachial route
Complications of brachial puncture for diagnostic arteriography are also said to be low, with a rate of up to 0.3% requiring surgery being reported.3 Minor complications not requiring surgery and resolving spontaneously have been reported as 8–15%.3,4
Other routes of access
Other routes of access are now much less commonly used. The axillary and high brachial routes were associated with a relatively high incidence of haematoma formation and occasional consequent nerve damage (up to 2.4%),5,6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree