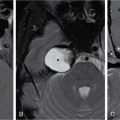
Madhavi Kandagaddala, Arunima Patra, Matthew Monachen The carotid space (CS) is a paired tubular space in the supra and infra-hyoid neck, encased by the carotid sheath. In surgical parlance, it is commonly referred to as the ‘post-styloid or retro-styloid parapharyngeal space’ or simply as the ‘carotid sheath’. Although abnormalities of the CS are less frequently encountered, they represent a formidable diagnostic and treatment challenge. The differentiation of a post-styloid lesion from a pre-styloid lesion is the foremost step in assessing and managing lesions in this region, as the contents of each space differ.The most common lesions in the CS are nerve sheath tumours and paragangliomas, followed by metastatic nodes and pseudo-lesions. This chapter will discuss the imaging approach to these lesions, with an emphasis on clinical relevance. The nomenclature of the suprahyoid CS has always been controversial. Historically, surgical and anatomic literature has been of the opinion that the suprahyoid CS is the same as the post-styloid parapharyngeal space (PPS). This is based on three observations: Some radiologists suggest that the carotid sheath and its contents (in the suprahyoid neck) can be considered a distinct ‘carotid space’. Although this may not necessarily reflect the underlying anatomy, it is of benefit to the radiologist, and hence the clinician in their ability to narrow the list of differential diagnoses of lesions that are localized to this space on imaging. The only disadvantage is that surgeons are more familiar with a pre-styloid/post or retro-styloid terminology. One acknowledges that what radiologists call the suprahyoid CS and surgeons call the post-styloid PPS are the same space. This chapter uniformly uses the term ‘post or retro-styloid carotid space’ or simply ‘carotid space’ for the suprahyoid and infra-hyoid extension of the carotid sheath and its contents. The CS extends from the jugular foramen-carotid canal above to the arch of the aorta below, i.e., from the skull base to the superior mediastinum. It is broadly divided into suprahyoid and infra-hyoid segments. The suprahyoid segment is further divided into the nasopharyngeal and oropharyngeal segments. The infra-hyoid segment likewise is split into the cervical and mediastinal segments (Fig. 3.14.1). These four segments are named after the region of the neck/mediastinum in which they lie. The natural boundary of the CS is the carotid sheath which receives a contribution from all three layers of the deep cervical fascia (Fig. 3.14.2). In the infra-hyoid neck, the sheath is more substantial and closely adherent to the vessels it contains. In the suprahyoid neck, however, it is less constant, as the middle layer of the deep cervical fascia of the neck is sometimes deficient, especially on the medial side. The contributions to the carotid sheath are as follows: As the CS spans the entire length of the neck, it is expected that its relations with the surrounding viscera will be different. The hyoid bone is at the approximate level of the bifurcation of the common carotid artery (Fig. 3.14.3). For convenience sake, the suprahyoid and infra-hyoid segments are considered separately. The relations are tabulated (Table 3.14.1) The contents of the CS can be easily recalled by remembering the extent of this space. As it extends from the jugular foramen–carotid canal above to the arch of the aorta below, by default it contains the structure exiting the jugular foramen and carotid foramen respectively. Exiting the jugular foramen is the internal jugular vein, the glossopharyngeal, vagal, and spinal accessory nerves. Exiting the carotid foramen is the internal carotid artery (ICA) with its accompanying sympathetic plexus. In addition to this, the hypoglossal nerve exits the adjoining hypoglossal canal and enters the CS just below the skull base. Embedded in the anterior wall of the carotid sheath is the Ansa cervicalis, a bundle of nerve fibres from the first three cervical spinal nerves. It is present only in the suprahyoid neck. The deep cervical lymph nodes accompany the neurovascular bundle. Given that this space is called the ‘carotid space’, the carotid artery is at the centre of the space with the Jugular vein postero-lateral to it, reflecting the approximate position of their respective foramina on the skull base. The 9, 12 and 11th cranial nerves leave the CS by piercing the anterior wall of the carotid sheath at the level of the soft palate to innervate the tongue and sternocleidomastoid muscle respectively; hence they are absent in the infra-hyoid CS. The vagus nerve that has to reach the mediastinum and beyond, traverses the entire length of the CS in the posterior notch formed between the carotid artery and internal jugular vein. The sympathetic plexus is not covered by the carotid sheath; hence it is not a true content of the CS; however, it courses close to the CS along its posterior and medial aspect (Fig. 3.14.8). To summarize, the contents of the CS are tabulated in Table 3.14.2. Lymph nodes – The middle and lower deep cervical lymph nodes (levels III and IV) are associated with, but not a content of the infra-hyoid neck CS. The nodes of the suprahyoid and infra-hyoid CS, as enumerated above, are known as the deep cervical nodes as they lie along the course of the Internal Jugular Vein on either side. Nodal masses can arise at any level in the CS and are part of the host of differential diagnoses of lesions of the CS. Nodal anatomy and pathology are dealt with in a separate chapter elsewhere in this textbook. Chemoreceptors – A small cluster of chemoreceptors and supporting sustentacular cells, known collectively as the carotid body, is located in the adventitia at the bifurcation of the common carotid artery. It is commonly accepted that it is not seen on routine imaging; however, carotid body tumours can arise from it and splay the internal and external carotid arteries. Glomus vagale arises from similar but distinct chemoreceptor cells located along the inferior ganglion of the vagus nerve. As the vagus nerve is located posteriorly, this tumor displaces the carotid arteries anteriorly without splaying the carotid bifurcation. The skull base chemodectomas arise from similar but distinct chemoreceptor cells situated along the Jacobsons nerve (Glomus tympanicum), Arnold’s nerve (Glomus jugulotympanicum) and Jugular bulb (Glomus Jugulare). KEY POINTS CS lesions arise from the structures within or closely related to the space, namely the carotid artery, internal jugular vein, lower cranial nerves, sympathetic plexus, paraganglion cells and lymph nodes. The most common masses in the CS are nerve sheath tumours and paragangliomas. The other primary CS pathologies include vascular abnormalities such as dissection, aneurysms & thrombosis and lymphadenopathy. In addition, normal vascular variants may mimic space-occupying lesions, and there may be a secondary invasion of the CS by abnormalities in the adjacent spaces. Abnormalities of the CS are enlisted in Table 3.14.3. The main approach to the lesions arising within the CS is to systematically narrow down the differentials and arrive at an appropriate and definitive diagnosis. It is therefore essential to identify the space of origin of the lesion (prestyloid vs. retrostyloid), followed by its morphology and the pattern of displacement of the vessels. Mass lesions originating in the retro-styloid space are closely related to the carotid artery and jugular vein and displace the adjacent structures in different directions depending on the level of the lesion. They displace the PPS fat and ICA anteriorly. In contrast, the pre-styloid tumors displace the PPS fat and ICA posteriorly (Fig. 3.14.9). Also, mass lesions in the retro-styloid space displace the styloid process anterior and lateral, the posterior belly of the digastric muscle and the parotid gland laterally. The retropharyngeal space and anterior compartment of the prevertebral space may also be encroached from a lateral to medial direction. The next step is to assess the size, solitary or multiple, location within the CS, internal morphology, that is solid or solid-cystic, vascularity, enhancement pattern, intracranial extension and associated bone changes. These features help in an accurate prediction of their histological diagnosis. The final crucial step is to assess the displacement pattern of the vascular structures within the CS. Tumours in the retro-styloid space are closely related to the carotid artery and jugular vein and displace them in different directions depending on the level and the structure of origin, providing clues to the origin of the lesion. Carotid body tumours cause widening and splaying of the internal and external carotid arteries. Tumours arising from the vagus nerve splay out the carotid arteries and jugular vein resulting in anterior and medial displacement of the CCA or ICA and posterior and lateral displacement of the IJV. Tumours arising from the cervical sympathetic chain displace both CCA or ICA, and IJV together anteriorly and laterally (Fig. 3.14.10). Imaging approach of the CS lesions is summarized in the flow chart (Fig. 3.14.11). Clinical evaluation of CS is quite limited, especially if lesions are small in size or located higher in the neck (close to skull base); hence imaging plays a key role in diagnosis and preoperative assessment and in surveillance. The main goals of imaging are summarized in Table 3.14.4. Several radiologic imaging and functional imaging techniques are available to evaluate CS lesions. The cross-sectional modalities are the primary anatomic imaging to identify their nature and stage these lesions. Isotope imaging is used to assess multiplicity and metastasis. Dynamic angiography is seldom performed, but it is valuable preoperatively for surgical planning and often for preoperative tumour embolization. Ultrasound neck is the first-line of examination for the investigation of neck swellings, particularly more useful in nonpulsatile lesions. A high frequency (above 7.5 MHz) linear transducer is ideal for assessing the CS lesions and their relation to the neck vessels. Greyscale ultrasound is used to differentiate between solid versus cystic lesion, delineation of tumour margins, its size and location. Doppler examination is useful for assessing lesion vascularity and helps differentiate paragangliomas from nodal metastasis, vessel thrombosis, vascular anomalies, and aneurysm. The role of ultrasound is restricted to lesions in infra-hyoid neck space. It has limited value in poorly accessible lesions located higher in the neck, close to the skull base. It is also of limited value for accurate assessment of the extent and origin in larger lesions and the evaluation of synchronous lesions. Based on the clinical and imaging information, a correct preoperative diagnosis can be made in 90%–95% of patients with a CS mass. Guided biopsy/FNAC is safe if meticulously done, and may be performed when imaging findings are atypical for paragangliomas or nerve sheath tumours and to confirm nodal metastasis. MRI with its high soft tissue resolution and non-ionizing nature is considered the imaging modality of choice for head & neck masses. However, long procedure time, costs, and limited availability in developing countries often make CT the imaging modality of choice, despite the use of ionizing radiation. Thin sections (2.5–3 mm) are acquired after contrast administration, from skull base to thoracic inlet in the axial plane and reformatted into coronal and sagittal sections. CT accurately and quickly assesses the location, extent, morphology, intra-lesional calcifications and bony destruction. The rate of contrast enhancement on dynamic CT (early filling and late equilibration phases) helps to differentiate paragangliomas from nerve sheath tumours. After a bolusi.v.injection of 100 mL of iodinated contrast at the rate of 1 mL/s, initial scan is performed within the first 10–20 seconds for the vascular filling phase, followed by repeat scanning for the equilibration phase at 60 s. Examination is best performed with 2.5 mm thick contiguous slices and a small field-of-view covering the region of interest. Attenuation versus time graphs depicting the sequential enhancement patterns of the tumors can also be plotted. Nerve sheath tumours have a paucity of veins and show delayed enhancement due to gradual accumulation of contrast in their extracellular space. On the other hand, paragangliomas show early enhancement due to rapid intravascular accumulation of contrast. Acquisition of routine CT only in the late phase will show a higher degree of enhancement in the otherwise hypo vascular neurogenic tumours, giving a false impression of paragangliomas. Therefore, the distinction between these two lesions based only on the degree of enhancement is difficult on the routine contrast-enhanced CT. CT angiography contributes to preoperative vascular assessment by visualizing the anatomical relations with the ICA, ipsilateral and contralateral venous return as well as tumours at other possible sites. MRI is the imaging modality of choice for characterization of CS lesions due to its superior contrast and soft-tissue resolution. It provides a more reliable assessment of the relation of the CS tumours with the vessels, relationship with the skull base, intracranial extension and detection of small tumours at other possible sites. MR protocol in the assessment of CS lesions is the multi-planar acquisition of the images in thin sections (3 mm) from skull base to thoracic inlet. The sequences acquired are T1-weighted (T1W), T2-weighted (T2W), Short Tau Inversion Recovery (STIR) and T1W fat-suppressed sequences, post-gadolinium fat-suppressed T1W sequence, and diffusion-weighted imaging. Conventional T1W and T2W sequences allow better delineation of the contour of a mass compared to CT. Smooth contours can be useful to differentiate benign lesions from the invasive margins of malignant paragangliomas or a vascular metastasis with extra-capsular spread. Infiltrative margins, tumour spread into adjacent spaces or low T2W signal can predict malignancy, and diffusion-weighted imaging further helps in differentiating benign from malignant lesions. Moderate-to large-calibre, rapid flow vessels associated with paragangliomas are easily identified by their flow voids, using flow-sensitive sequences or by MR angiography (phase contrast, 3D TOF or gadolinium-enhanced). Non-invasive CT and MR angiogram have replaced conventional arteriography as the primary diagnostic tool for tumour vascularity. Hence, conventional arteriography is no longer indicated for the diagnosis of paraganglioma, but is reserved for preoperative endovascular embolization to minimize intraoperative blood loss or for preoperative balloon occlusion test. It may show dilated feeder arteries and rapid venous return. Although it may be less often used for carotid body tumour because of concern of reflux of embolization material into the ICA, but is performed much more frequently for very large paragangliomas of the vagus nerve or lesions close to the skull base. Conventional angiography for carotid body tumours demonstrates the ‘Lyre sign’ due to splaying of the internal and external carotids arteries. It is complementary to radiological imaging for paragangliomas and provides specific information about local staging, multifocal lesions, metastasis and assessment of post-treatment residual or recurrent lesions. This is due to expression of specific somatostatin receptors by the paragangliomas, which other neck lesions such as nerve sheath tumours lack. Paragangliomas are somatostatin receptor (SSR-2) expressing neuroendocrine tumours. 68Ga-Labeled Somatostatin Analogue PET-CT is the radiotracer of choice, with the highest sensitivity (close to 100%) for the detection of small lesions and multifocal forms not visible on morphological imaging. It is superior to other radiotracers, especially for multiple small-sized lesions. In absence of available 68Ga-DOTA PET, a combination of 18F-FDOPA and 18F-FDG PET/CT is recommended, especially for patients with multifocal tumours. There is a promising role for using 177Lu-DOTATATE for both diagnosis and highly targeted radionuclide therapy of these tumours. KEY POINTS – IMAGING IN CAROTID SPACE LESIONS
3.14: Carotid space
Introduction
History
Anatomy
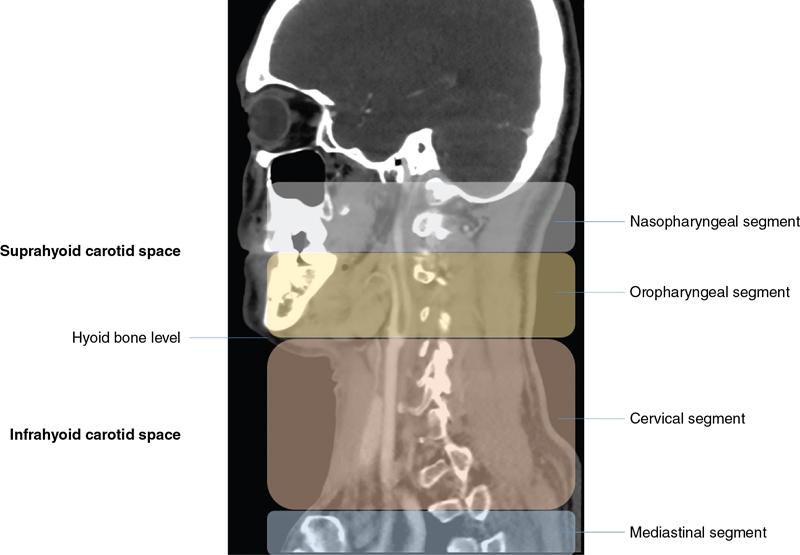
Extent and division
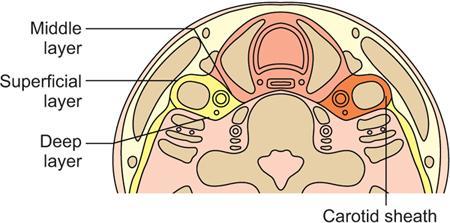
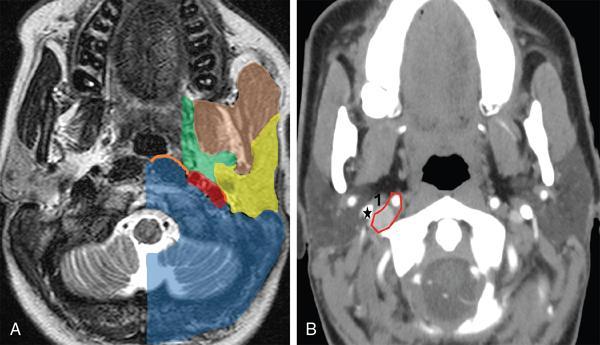
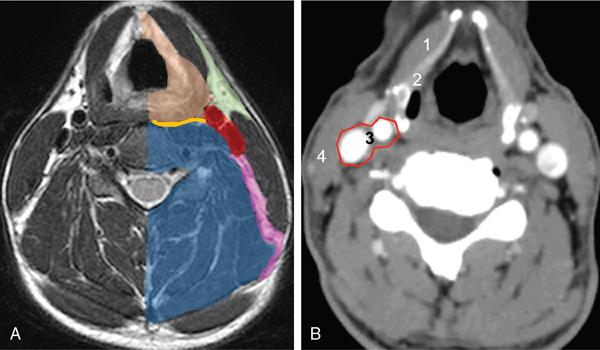
Boundaries
Relations
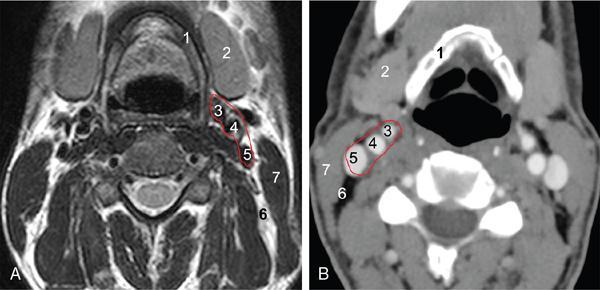
Suprahyoid Carotid Space (Figs 3.14.4 and 3.14.5)
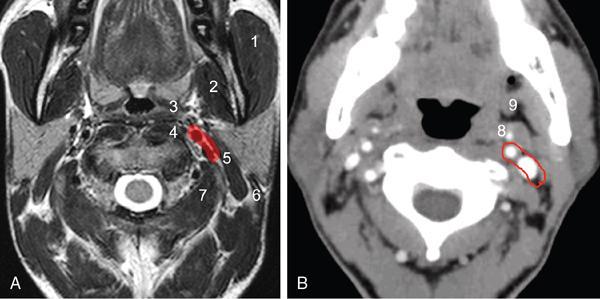
Infra-Hyoid Carotid Space (Figs 3.14.6 and 3.14.7)
Anterior
Masticator space, parapharyngeal space
Anterior cervical space
Posterior
Peri vertebral space
Peri vertebral space
Medial
Retropharyngeal space
Retropharyngeal and visceral space
Lateral
Parotid space
Posterior cervical space
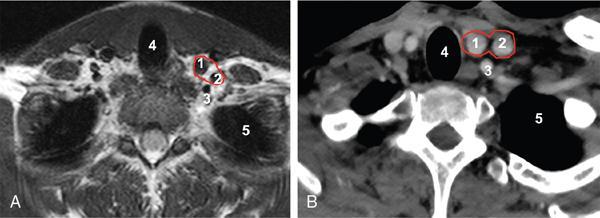
Contents
Suprahyoid Neck
Infra-Hyoid Neck
Arteries
Internal carotid artery
Internal carotid arteryExternal carotid artery
Veins
Internal jugular vein
Internal jugular vein
Nerves
Cranial nerves IX, X, XI, XII
Cranial nerve X
Nodes
Upper deep cervical lymph nodes (level II)
Middle and lower deep cervical lymph nodes (levels III and IV)
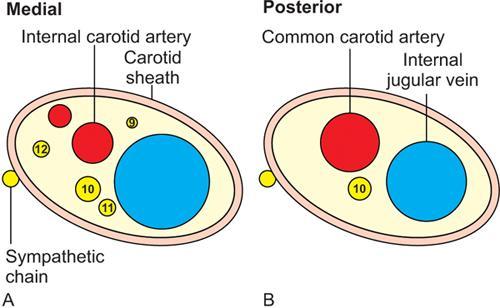
Sympathetic plexus
Not encased by the carotid sheath. It runs posterior and medial to the carotid space
None
Imaging approach and imaging modalities
Origin
Pathologies
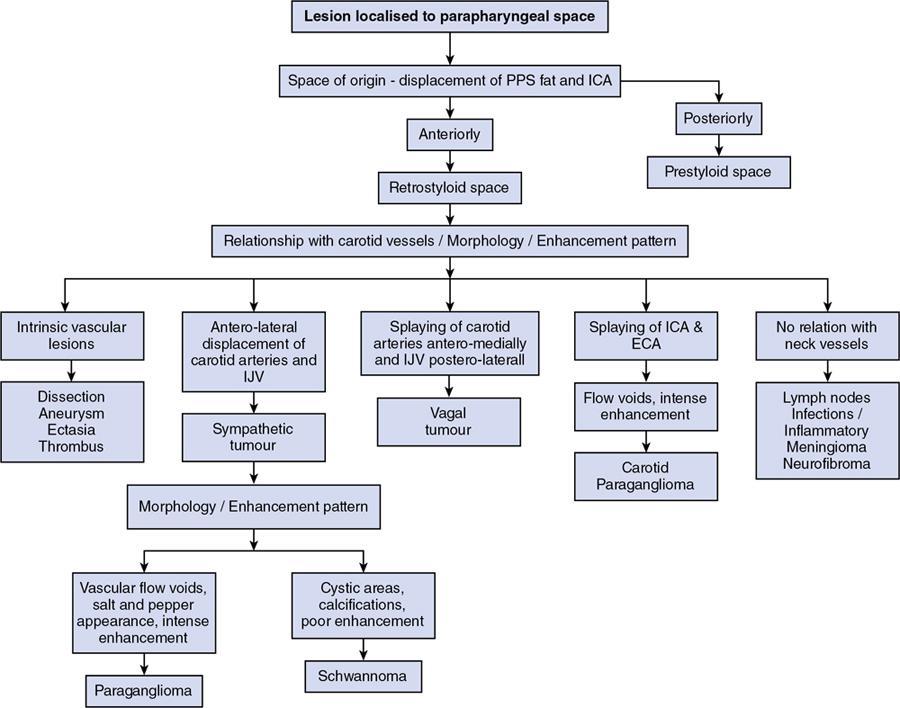
Imaging approach
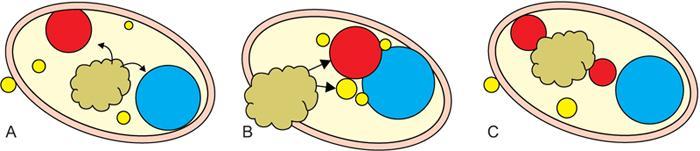
Step 1: Space of origin of the lesion: Retro-styloid vs. pre-styloid space
Step 2: Morphology of the lesion
Step 3: Displacement of the vascular structures
Goals of imaging of the CS lesions.
Role of imaging modalities
Ultrasound (USG)
Ultrasound-guided transcervical fine needle aspiration (FNA) cytology or biopsy.
Computed tomography (CT)
Dynamic CT.
Magnetic resonance imaging (MRI)
Conventional angiography
Radionuclide imaging
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Carotid space