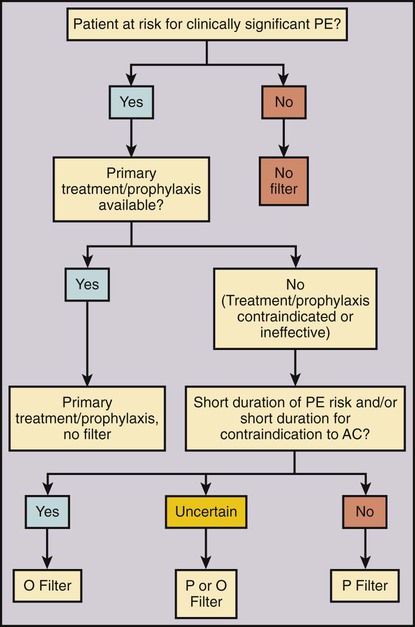
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality. The most feared condition is pulmonary embolism (PE) resulting from blood clot dislodging from the lower extremity or pelvis, and less commonly from the upper extremity, into the lungs. Symptomatic PE is diagnosed in about 355,000 patients per year in the United States and results in 240,000 deaths annually.1 Anticoagulant medication is the current accepted first-line treatment and prophylaxis for PE.2 However, in the case of a massive PE, the right side of the heart can become acutely overloaded, leading to right ventricular dysfunction, hemodynamic compromise, and possibly cardiac arrest and death. Anticoagulation alone is often insufficient for these severely ill patients.3 Thrombolytic therapy4 or a combined mechanical and fibrinolytic treatment for massive PE has been described, with promising outcomes.5 A different and likely more efficient approach is to prevent a symptomatic PE before it happens by capturing the clot on its way to the pulmonary circulation. This idea of PE prevention is more than 100 years old. In 1874, John Hunter described femoral vein ligation to prevent PE.6 In the mid-1940s, inferior vena cava (IVC) ligation was proposed to include capturing clot coming from the pelvic veins.7 IVC ligation was effective in preventing PE but associated with a 14% operative mortality rate and a 33% chance of chronic venous stasis.8 Ligation was therefore replaced by compartmentalization of the IVC with sutures, staples, or clips, which reduced the venous stasis problem, but unfortunately not the operative mortality rate (12%).8 To reduce operative mortality, the first percutaneous filter, the Mobin-Uddin umbrella, was introduced in 1967.8 Use of the Mobin-Uddin device, which had the design of a reversed cone, was stopped because of a high IVC occlusion rate of 60%.8 The cone-shaped Greenfield filter marked the start of the era of permanent IVC filters and is still in use. The first percutaneous placement of a Greenfield filter was reported in 1984.9 Since then, many different devices designed for percutaneous insertion have been developed. Despite widespread availability and relative ease of use, the frequency of use varies substantially among different countries and even within the United States. There is little doubt that caval filtration reduces the risk of PE. The only randomized study comparing anticoagulation alone versus anticoagulation with use of an IVC filter showed a significantly lower rate of symptomatic PE in the filter group of 3.4% versus 6.3% without filter at 2 years10 and 6.2% versus 15.1% at 8 years.11 The same patient population demonstrated an increased risk of deep venous thrombosis (DVT) at 2 years with 20.8% with a filter in place compared with 11.6% without a filter.10 This difference continued at 8 years: 35.7% with filter versus 27.5% without.11 In the same patient population, anticoagulation showed a substantial risk for bleeding, with 3.75% major events at 12 days and 10.3% at 2 years.10 Because of the lack of good scientific data, the benefit, risk, and cost of IVC filters compared with standard anticoagulation therapy are assessed rather subjectively, leading to very different usage of IVC filters around the world. The simple idea of an IVC filter is to prevent a PE. The indication for caval filtration has two components: (1) the presence or a high risk of a PE paired with a contraindication, complication, or insufficiency or (2) a high risk of anticoagulation, which is the primary treatment for VTE. One without the other is not enough to place an IVC filter. In an attempt to standardize use of IVC filters, a multidisciplinary consensus conference led by the Society of Interventional Radiology (SIR) described an algorithm for IVC filter placement (Fig. 104-1). Depending on the risk of PE and/or downsides of anticoagulation, the indications for IVC filters can be categorized in three groups: absolute indications, relative indications, or prophylactic indications (Table 104-1). Absolute indications are situations with proven VTE when anticoagulation fails or if anticoagulation led to a bleeding complication or is considered contraindicated. The latter group of patients includes those with an active internal hemorrhage, a hemorrhagic stroke, severe bleeding diathesis, recent major surgery including neurosurgical procedures, or an intracranial neoplasm. TABLE 104-1 Indications for Inferior Vena Cava Filters From Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: Report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol 2006;17:449–59. Although there is little controversy about the absolute indications, there are substantial differences about the relative and prophylactic indications. A large free-floating thrombus can look worrisome on imaging, and placement of a filter intuitively seems to be the right thing. However, in a prospective study, no difference in recurrent PE was found between free-floating and occlusive DVT in patients treated with intravenous unfractionated heparin.12 Other patients with limited cardiopulmonary reserve may not tolerate any further PE and therefore benefit from an IVC filter. The problem in these cases is to define what the critical cardiopulmonary reserve is. The same subjectivity applies for the assessment of increased risks of anticoagulation for patients at higher risk to fall. Besides the medical issues of anticoagulation, patient compliance plays an important role for either oral anticoagulation or subcutaneous low-molecular-weight heparin injection therapy. Therefore, it seems reasonable to place a filter to protect noncompliant patients from PE. Prophylactic IVC filter placement has been proposed for patients with a high risk for VTE undergoing spinal surgery,13 open gastric bypass surgery with a body mass index greater than 55 kg/m2, or severe trauma.14,15 Trauma patients were considered at high risk if they had one of the following injuries: spinal cord injury with neurologic deficit, severe fractures of the pelvis and/or long bone, or severe head injury. Another study showed a benefit of prophylactic filter placement within 48 hours compared with delayed filter placement in the presence of VTE.16 All these mentioned studies used permanent IVC filters.13,16 A new dimension was added to the discussion about prophylactic filter placement by the introduction of optional filters. Optional filters are permanent filters with the option to be removed or converted to a stent if caval filtration is no longer needed. The concept of removing an IVC filter if it is no longer needed is based on the randomized trial by Decousus et al., who showed an early reduction of PE within the filter group with an increased DVT rate after 2 years.10 The SIR guidelines for optional filters state that “there is no unique new indication for optional filters distinct from permanent IVC filters,”17 but the option to retrieve a filter and therefore avoid possible long-term complications has somewhat changed the risk/benefit equation of IVC filters. Optional filters are most beneficial if the PE risk and/or the contraindication to anticoagulation is limited to a short time period (see Fig. 104-1). Retrievable filters successfully acted as protection devices and prevented symptomatic PE during catheter-directed thrombolysis in 31 consecutive patients.18 Because of the potential of preventing a large PE during thrombolysis and a relative low risk of retrievable filters, more and more interventionalists are using retrievable filters during thrombolysis. Optional filters are also increasingly used in trauma patients at high risk of VTE. Retrievable filters have been shown to be safe, prevent fatal PE, and effectively provide a bridge to anticoagulation.19 However, a critical comment was made that relatively few of these retrievable filters were actually removed.20 In a retrospective study of a level 1 trauma registry, “only” 35% of filters were retrieved.20 In a different publication, only 14% of IVC filters with a temporary indication for caval filtration were retrieved.21 Because of these surprisingly low retrieval rates, specific indications for retrievable IVC filters should be considered cautiously. In addition to medical concerns, there have also been legal issues in the United States around the problem of leaving retrievable filters in place. There are very few contraindications for caval filtration. One contraindication for an IVC filter is lack of a location to place the filter. Examples would be occlusion or absence of the IVC. In these conditions, blood returns to the heart through a collateral venous network that consists typically of multiple tortuous and small veins preventing large blood clots from traveling to the pulmonary circulation. However, if a large collateral venous pathway develops over time, placement of a filter into the azygos or hemiazygos vein can be considered.22 Another contraindication to percutaneous IVC filter placement is lack of access to the IVC in cases when all possible access sites—jugular, subclavian, and femoral veins—are occluded. Uncontrollable hemostasis is a contraindication for percutaneous procedures in general. However, because of the relatively small insertion profile (as low as 6F), the availability of jugular delivery systems, and the availability of ultrasound-guided venous puncture, IVC filters can be safely placed in almost every patient. Other medical conditions that could interfere with IVC filter placement include allergy to iodinated contrast agents and/or renal insufficiency. Alternative contrast agents (gadolinium or CO2) can be used in these patients, allowing safe filter placement.23,24 CO2 venography before filter placement is safe and accurate in the majority of patients and underestimated IVC diameter in only 3.3% (IVC diameter thought to be below 28 mm when in fact it was above 28 mm).24 Underestimating IVC diameter can have an impact on certain IVC filters that are approved to a maximal IVC diameter of 28 mm. The features of all available U.S. Food and Drug Administration (FDA)-approved IVC filters are summarized in Table 104-2. For a more detailed discussion of different filter types and new developments please see Chapter 12. TABLE 104-2 U.S. FDA-Approved Inferior Vena Cava Filters (2011) FDA, Food and Drug Administration; ID, inner diameter; U.S., United States.
Caval Filtration
Indications
Absolute Indication (Proven VTE)
Relative Indications (Proven VTE)
Prophylactic Indication (No VTE, High Risk for Anticoagulation)
Recurrent VTE despite adequate anticoagulation
Iliocaval DVT
Trauma patient with high risk of VTE
Large, free-floating thrombus
Surgical procedure in patient at high risk of VTE
Contraindication to anticoagulation
Thrombolysis for iliocaval DVT
Medical condition with high risk of VTE
Complication of anticoagulation
VTE with limited cardiopulmonary reserve
Inability to achieve/maintain therapeutic anticoagulation
Poor compliance with anticoagulation
High risk of complication of anticoagulation (e.g., frequent falls)
Contraindications
Equipment
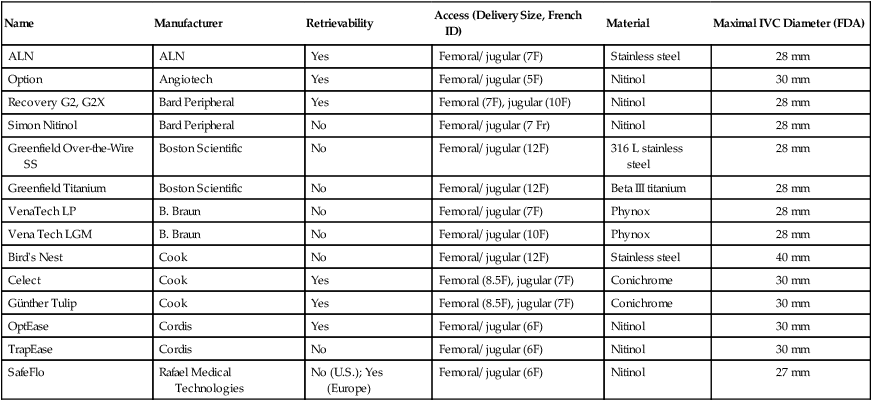
Name
Manufacturer
Retrievability
Access (Delivery Size, French ID)
Material
Maximal IVC Diameter (FDA)
ALN
ALN
Yes
Femoral/ jugular (7F)
Stainless steel
28 mm
Option
Angiotech
Yes
Femoral/ jugular (5F)
Nitinol
30 mm
Recovery G2, G2X
Bard Peripheral
Yes
Femoral (7F), jugular (10F)
Nitinol
28 mm
Simon Nitinol
Bard Peripheral
No
Femoral/ jugular (7 Fr)
Nitinol
28 mm
Greenfield Over-the-Wire SS
Boston Scientific
No
Femoral/ jugular (12F)
316 L stainless steel
28 mm
Greenfield Titanium
Boston Scientific
No
Femoral/ jugular (12F)
Beta III titanium
28 mm
VenaTech LP
B. Braun
No
Femoral/ jugular (7F)
Phynox
28 mm
Vena Tech LGM
B. Braun
No
Femoral/ jugular (10F)
Phynox
28 mm
Bird’s Nest
Cook
No
Femoral/ jugular (12F)
Stainless steel
40 mm
Celect
Cook
Yes
Femoral (8.5F), jugular (7F)
Conichrome
30 mm
Günther Tulip
Cook
Yes
Femoral (8.5F), jugular (7F)
Conichrome
30 mm
OptEase
Cordis
Yes
Femoral/ jugular (6F)
Nitinol
30 mm
TrapEase
Cordis
No
Femoral/ jugular (6F)
Nitinol
30 mm
SafeFlo
Rafael Medical Technologies
No (U.S.); Yes (Europe)
Femoral/ jugular (6F)
Nitinol
27 mm
Radiology Key
Fastest Radiology Insight Engine