This review highlights the 2 major molecular imaging modalities that are used in clinics, namely single-photon emission computed tomography (SPECT) and positron emission tomography (PET), and their added value in management of patients with brain tumors. There are a variety of SPECT and PET radiotracers that can allow imaging of different molecular processes. Those radiotracers target specific molecular features of tumors, resulting in improved specificity of these agents. Potential applications include staging of brain tumors and evaluating post-therapeutic changes.
Key points
- •
Molecular imaging provides functional information based on target detection, which can be helpful in management of brain tumors.
- •
There are multiple different radiotracers developed based on different mechanisms that can be used in neuro-oncology.
- •
These radiotracers can detect brain tumors, differentiate low-grade and high-grade lesions, determine eligibility for theranostics, estimate prognosis, and evaluate post-radiation changes.
Introduction
Brain neoplasms include a diverse group of primary tumors as well as secondary tumors or metastases from neoplasms outside the central nervous system (CNS), which occur more frequently than the primary brain tumors. The incidence rate of primary CNS tumors in adults in the United States is approximately 30 per 100,000 population, with approximately one-third of these tumors being malignant.
Single-photon emission computed tomography (SPECT) and positron emission tomogarphy (PET) are the 2 major tomographic modalities in nuclear medicine that provide functional information regarding different tissues and disorders. Different nuclear medicine radiotracers are utilized for evaluation of brain neoplasms. Although most novel emerging molecular imaging radiotracers are compatible with PET imaging, SPECT remains an alternative modality with lower costs and wider availability.
The emergence of novel radiotracers provides major opportunities in the diagnosis and management of brain tumors. This review delineates the complementary role of conventional and novel molecular imaging radiotracers in neuro-oncology. Most emerging radiotracers are PET agents and, therefore, this review largely focuses on PET imaging agents.
Single-photon emission computed tomography
SPECT imaging provide a reasonable imaging alternative to PET, with higher affordability and availability. In SPECT, the gamma camera rotates about the patient and acquires projections at different angles ( Fig. 1 ). The main limitation of SPECT imaging is lower resolution and lack of precise anatomic details compared to PET imaging. Recent development of integrated hybrid SPECT/computed tomography (CT), however, has improved the localization of tumor and increased the diagnostic accuracy.

Technetium-99m or [ 99m Tc] labeled compounds and thallium-201or [ 201 Tl] are 2 example of SPECT radiotracers that have been evaluated in brain tumors. [ 99m Tc] labeled compounds are preferred over [ 201 Tl] based on better contrast resolution; less radiation to the patient; universal availability; and favorable gamma emission characteristics of [ 99m Tc], including the 140-keV gamma ray energy and high photon flux compared to [ 201 Tl].
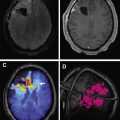
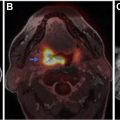
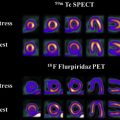
[ 99m Tc]methoxy-2-isobutylisonitrile ([ 99m Tc]MIBI) and [ 99m Tc]tetrofosmin ([ 99m Tc]TF) are passively accumulated in mitochondria and are considered markers of cellular transmembrane electrical potentials. These features result in higher uptake in neoplastic cells in comparison with surrounding tissues. [ 99m Tc]MIBI and [ 99m Tc]TF can help in improving diagnostic and prognostic accuracy of brain tumors. Alexiou and colleagues showed that [ 99m Tc]TF can provide helpful information to differentiate recurrence from radiation necrosis , with the best results observed in gliomas, particularly when anatomic imaging is equivocal ( Fig. 2 ). Semiquantitative analysis of [ 201 Tl] uptake can differentiate tumor recurrence and postradiation necrosis in metastatic brain tumors. Novel SPECT radiotracers are under development for detection of brain tumors. For example, diethylenetriamine pentaacetic acid–conjugated CooP with indium-111 can detect gliomas in 85% of mice.

Positron emission tomography
PET agents play a crucial role in understanding the pathophysiology of neoplasms, developing targeted therapies, and monitoring response to therapy. These agents play a valuable role in diagnosis, classification, staging, image-guided therapy planning, and post-therapeutic evaluation of brain tumors. Table 1 summarized different PET radiotracers in neuro-oncology. Current and emerging novel radiotracers are discussed within the context of their mechanisms of action.
| Tracer | Mechanism | Tumor Type |
|---|---|---|
| [ 11 C]acetate | Anabolism by acetyl-CoA synthesis, cell membrane | Glioma, meningioma, meningioma, schwannoma, metastases |
| [ 11 C]CHO | Phospholipids synthesis | Glioma, CNS metastases, meningioma, schwannoma |
| [ 11 C]MET | Amino acid uptake (methionine based) | Glioma, germinoma, CNS metastases, meningioma, CNS lymphoma |
| [ 18 F]FDOPA | Amino acid uptake | Glioma, CNS metastases, meningioma |
| [ 18 F]FAZA | Hypoxia | High-grade glioma |
| [ 18 F]FDG | Glucose metabolism | Glioma, CNS metastases, meningioma, CNS lymphoma, oligodendroglioma |
| [ 18 F]FET | Amino acid uptake (tyrosine based) | Glioma, CNS metastases, meningioma, medulloblastoma, ganglioglioma |
| [ 18 F]FMISO | Hypoxia | Glioma |
| [ 68 Ga]DOTA-TATE | somatostatin receptor up-regulation | Meningioma, hemangioblastoma, CNS neuroendocrine, medulloblastoma |
| [ 68 Ga]PSMA | PSMA | Glioma, metastasis, meningioma |
Tracing glucose metabolism
2-Deoxy-2-[ 18 F]fluoro- d -glucose
2-Deoxy-2-[ 18 F]fluoro- d -glucose ([ 18 F]FDG) is the most commonly used PET radiotracer in oncology. Its half-life is relatively longer than most other positron emitters (approximately 2 hours) and it is widely and readily available. Increased glucose metabolism is associated not only with growth but also with malignant transformation.
The Response Assessment in Neuro-Oncology working group recently provided recommendations in using glucose and amino acid radiotracers in glioma as a complementary modality to MR imaging. To improve the accuracy of [ 18 F]FDG PET in detecting brain tumors, two critical steps are recommended. The first step is to coregister the images with MR imaging if a PET/CT was performed, and the second is to acquire delayed images to improve differentiation of brain neoplasm from cortical uptake. PET/MR imaging provides simultaneous data acquisition which results in gathering functional and anatomic data with significant improvement of spatial and temporal resolution. Few institutions have dedicated PET/MR imaging scanners, however, and, therefore, the importance of fusion software is critical.
2-Deoxy-2-[ 18 F]Fluoro- d -Glucose PET in Neuro-Oncology
Delineating tumor boundaries
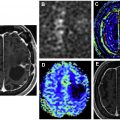
One of the challenges in neuro-oncology is delineation of the tumor margins at the edge of gray matter, which also intrinsically shows high activity. This can be addressed with coregistration with MR imaging or delayed PET imaging ( Fig. 3 ). Tumor demonstrates higher [ 18 F]FDG avidity than gray matter on delayed acquisitions due to lower degradation of intracellular [ 18 F]FDG-6-phosphate in tumor than in brain.

Tumor grading
Some studies have shown that [ 18 F]FDG PET can differentiate low-grade and high-grade gliomas based on glioma–to–white matter and glioma–to–gray matter ratios. Tumor–to–white matter ratio of greater than 1.5 and tumor–to–gray matter ratio of greater than 0.6 can differentiate high-grade from low-grade gliomas, with 94% sensitivity and 77% specificity, although other studies have failed to differentiate high-grade and low-grade gliomas based on [ 18 F]FDG uptake.
Determining prognosis
Higher [ 18 F]FDG uptake in a previously known low-grade glioma can indicate anaplastic transformation. [ 18 F]FDG PET can predict survival regardless of histologic classification. Some studies show that [ 18 F]FDG uptake is the most powerful predictor of progression-free survival and overall survival in comparison with other variables, such as histologic grade.
Guiding post-therapy evaluation
One of the major advantages of [ 18 F]FDG PET compared with structural imaging is differentiating radiation necrosis from recurrent disease. This feature is more reliable in gliomas in contrast to metastatic disease. A meta-analysis regarding the diagnostic accuracy of [ 18 F]FDG PET in detecting recurrences in gliomas showed heterogeneous diagnostic accuracies across the studies, with sensitivity of 0.77 (95% CI, 0.66–0.85) and specificity of 0.78 (95% CI, 0.54–0.91). [ 18 F]FDG PET can be used in planning for stereotactic biopsy by improving delineation of higher-grade foci within a heterogeneous tumor. This improves the diagnostic yield of brain biopsy.
Amino acid metabolism
Amino acids are required in the synthesis of proteins and can indicate level of metabolism and proliferation. Amino acids generally demonstrate a low background uptake in gray matter and white matter, which improves tumor-to-background ratio (TBR). TBR is used to compare the uptake in tumor to the contralateral lobe. The uptake of amino acid–based radiotracers in tumoral cells is based on overexpression of amino acid transporters, which correlates with malignant phenotype and angiogenesis.
Methyl-[ 11 C]- l -Methionine
The most commonly used radiolabeled amino acid in detecting brain tumors is methyl-[ 11 C]- l -methionine ([ 11 C]MET). The relatively short half-life of carbon-11 ([ 11 C]MET), approximately 20 minutes, limits its usage to institutions with an on-site cyclotron. This radiotracer can be synthesized rapidly without requirement for purification. The intracellular uptake happens through transmembrane transport by sodium independent L-transporter based on a concentration gradient. [ 11 C]MET brain uptake generally is lower than brain tumors, resulting in high detection rate and sharp delineation of the tumor.
[ 11 C]MET PET can detect primary gliomas with high sensitivity and specificity, with sensitivities ranging from 76% to 100%. Besides neoplasms, however, inflammatory cells have increased uptake, making this radiotracer an unideal method in differentiating tumor from inflammation.
The results of quantitative analysis among different [ 11 C]MET PET studies have been controversial in grading primary gliomas. Low-grade gliomas generally show lower uptake in contrast to high-grade gliomas. A meta-analysis in 2019 showed that [ 11 C]MET PET can differentiate low-grade from high-grade gliomas with higher sensitivity than [ 18 F]FDG PET.
Some studies have shown that [ 11 C]MET PET can be used as a prognostic tool with higher uptake values associated with worse outcome. For example, Nariai and colleagues showed significant correlation between survival and pretreatment TBR. In contrast, Ceyssens and colleagues showed that [ 11 C]MET PET does not predict survival in patients with brain tumors.
[ 11 C]MET PET has been shown to improve tumor delineation with higher accuracy in comparison to CT or MR imaging and can identify areas with highest risk of recurrence. This radiotracer can improve biopsy planning by identifying the regions with higher grades and determining better target regions in contrast to structural imaging modalities.
O-(2-[ 18 F]fluoroethyl)- l -Tyrosine
O-(2-[ 18 F]fluoroethyl)- l -tyrosine ([ 18 F]FET) is a tyrosine analogue that can be used in detection of brain tumors. This molecule is not incorporated into proteins; hence, it has greater intracellular stability in contrast to other amino acid radiotracers.
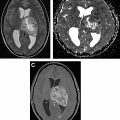
[ 18 F]FET PET can diagnose brain tumors and grade gliomas with higher sensitivities than [ 18 F]FDG. It can differentiate low-grade from high-grade gliomas as well as low-grade and high-grade meningiomas based on TBR. In untreated metastatic lesions, [ 18 F]FET PET uptake is increased in only two-thirds of lesions less than 1.0 cm whereas all lesions greater than 1.0 cm show pathologic uptake independent of tumor size. Fig. 4 shows [ 18 F]FET uptake in metastatic melanoma lesions, which demonstrate high variability.

This radiotracer can be helpful for target selection and biopsy guidance as well as tumor resection planning. A cost-effectiveness analysis showed that [ 18 F]FET PET and MR imaging may be superior to MR imaging alone in determining the biopsy site in glioma.
[ 18 F]FET also is useful in detection of residual brain tumor after surgery. Early evaluation of the resection status in high-grade glioma is feasible with [ 18 F]FET PET, and PET findings have been shown to correlate with intraoperative assessment with 5-aminolevulinic acid as well as MR imaging results. Multiple studies have shown the role of [ 18 F]FET PET in differentiating recurrence from post therapeutic changes. In a related study, Galldiks and colleagues concluded that [ 18 F]FET PET parameters can differentiate progressive or recurrent glioma from post treatment changes with higher accuracy than MR imaging.
[ 18 F]FET PET also can predict prognosis and survival in gliomas. Early time to peak in dynamic [ 18 F]FET PET is associated with worse outcome in newly diagnosed high-grade gliomas. Maximum and mean TBRs are significant and independent predictors for progression-free survival and overall survival.
l-3,4-Dihydroxy-6-[ 18 F]fluoro-phenyl-alanine
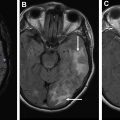
l-3,4-Dihydroxy-6-[ 18 F]fluoro-phenyl-alanine ([ 18 F]FDOPA) is transported into cells through large amino acid transporter and then is decarboxylated by DOPA decarboxylase to [ 18 F]dopamine that is trapped intracellularly in storage granules by vesicular monoamine transporters. Chen and colleagues compared [ 18 F]FDOPA with [ 18 F]FDG PET in 81 patients with brain tumor. [ 18 F]FDOPA had higher sensitivity (96%) in identifying brain tumors compared with [ 18 F]FDG (61%), with similar specificities (43%). [ 18 F]FDOPA is more accurate than [ 18 F]FDG PET for diagnosing low-grade tumors and evaluating recurrent tumors ( Fig. 5 ). Moreover, it was able to distinguish tumor recurrence from radiation necrosis.