Fungal infections of the central nervous system range from chronic indolent forms to acute fulminant forms causing significant morbidity and mortality. They often show atypical and variable neuroradiologic findings because of the absence of typical inflammatory response. The neuroradiologist must have high degree of suspicion in immunocompromised patients regarding the possibility of central nervous system fungal infections and keep in mind the appearances of various fungi even when immune response is intact. Next is to identify the pattern of involvement whether hematogenous or direct sinonasal and then make a well-informed speculation regarding the type of the pathogen based on the clinical features and imaging appearance.
Fungal infections of the central nervous system (CNS) are rare although relatively more frequent in the tropics. Fungi can be grouped broadly into filamentous fungi, dimorphic fungi, and yeasts. Clinically common fungi that invade the CNS, such as Aspergillus and Mucor , are classified as filamentous fungi. Cryptococcus and Candida are yeasts. Other fungi, such as Blastmyces , Histoplasma , Coccidioides , and Paracoccidioides , fall under dimorphic fungi.
CNS fungal infections occur mostly in immunocompromised patients, especially those with HIV infection and those with solid-organ transplantation. In the general population, however, CNS fungal infections are seen more frequently in patients with long-standing diabetes. These infections are generally secondary in nature, with the primary focus elsewhere in body. Primary CNS fungal infections are rare.
The region of brain affected by a particular fungus largely depends on its size, which in turn decides the site of lodgment in the brain. The smaller fungi, such as Candida and Cryptococcus , can reach the cerebral microcirculation causing meningitis and microabscesses. The larger ones, such as Aspergillus and Mucor , occlude the large arteries or their major branches resulting in large areas of bland infarction. These areas of infarction then secondarily may change into abscesses.
Most people affected by fungal infections, being immunocompromised, lack cellular reactions and thus neuroimaging findings are often nonspecific. These patients may manifest with a host of features pointing toward underlying chronic meningitis, acute meningoencephalitis brain abscesses, or stroke. The correct diagnosis of fungal infections thus is based on combined approach of imaging, clinical settings, and cerebrospinal fluid findings.
Although almost any fungus may cause encephalitis, those common to the tropics are cryptococcal meningoencephalitis, aspergillosis, mucormycosis, and more rarely candidiasis. Other fungal infections, such as blastomycosis, coccidioidomycosis, and histoplasmosis, are not generally seen in tropics.
Common imaging features
Fungal abscesses appear as solid or ring-enhancing lesions. MRI shows patchy or punctate T2-hyperintensity with frequently absent enhancement on postcontrast images. Conventional MRI may show similar features in the pyogenic, tubercular, and fungal abscesses. On diffusion-weighted imaging (DWI), low apparent diffusion coefficient (ADC) has been found in fungal similar to pyogenic abscesses. More specifically, the fungal abscesses may show restriction of diffusion in the intracavitary projections and the wall, with center of the abscess showing no restriction of diffusion.
On MR spectroscopy fungal lesions are known to show lipids (1.2–1.3 ppm), lactate (1.3 ppm), alanine (1.5 ppm), acetate (1.9 ppm), succinate (2.4 ppm), and choline (3.2 ppm), and unidentified multiple signals are also seen between 3.6 and 3.8 ppm. These peaks are caused by trehalose sugar present in the fungal wall.
Specific fungal infections
Aspergillosis
Aspergillus is a ubiquitous saprophytic fungus found in soil and plants. It has regularly septate hyphae and produces spores. Individuals are infected by inhaling these spores, which settle in lungs and paranasal sinuses.
Invasive aspergillosis is an increasing problem in patients treated with intensive chemotherapy and immunosuppressive therapy after solid organ or bone marrow transplantation. Intracranial spread of aspergillosis occurs in 10% to 20% of cases.
Two forms of Aspergillus infections in the brain have been recognized. The rhinocerebral form results from direct spread of fungi from paranasal sinuses into the brain. Cerebrovascular aspergillosis results from hematogenous spread of the fungi leading to occlusion of large and middle-size arteries. MRI features of both are quite varied.
Imaging features
Imaging studies of patients with cerebral aspergillosis reveal three general patterns: (1) single or multiple infarcts; (2) ring lesions (single or multiple) consistent with abscess formation; and (3) dural or vascular infiltration arising from the paranasal sinuses or orbits. Other findings on imaging include mycotic aneurysm and contrast enhancement of affected parenchyma, and hemorrhagic transformation of infarcted areas.
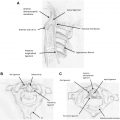
In the hematogenous type of spread because of angioinvasive character of the fungi, early manifestations include ischemic lesions, which are detected by using DWI. Involvement of the basal ganglia is a characteristic finding, indicating a predominant affection of the lenticulostriate and thalamoperforator arteries ( Fig. 1 ). A cerebral infarction is followed in most cases by a conversion to an infected area, with associated abscess formation.
Fungal cerebritis lesions are nonenhancing and are usually located in the basal ganglia and deep white matter. Peripheral rim enhancement has been reported in mature pyogenic and fungal abscesses. In pyogenic and tubercular abscesses, the outer margin of the wall is either smooth or lobulated in contrast to the fungal lesions, which have a crenated wall in most cases.
In one study, fungal abscesses showed nonenhancing intracavitary projections that were not seen in other types of abscesses. The DWI images and ADC values showed restricted diffusion in these projections and the wall of the fungal abscess ( Fig. 2 ). The study inferred that the presence of irregular projections in abscesses with ADC values less than those of the wall is probably because of the presence of fungal elements containing paramagnetic substances. One uncommon complication of vascular wall erosion is fungal vasculitis, which may lead to mycotic aneurysm formation.
In the Rhinocerebral pattern of spread, initial involvement of the paranasal sinuses and the orbits exists, which presents in most cases as a nonspecific, contrast-enhancing mucosal thickening of the sinuses on CT or MRI. A serious complication of this localized infection is the infiltration and erosion of the adjacent bony structures leading to localized meningitis.
In the brain, a well-defined granuloma is usually seen in the basifrontal or anterior temporal region often having continuity with the adjacent sinus disease. The gramulomas are virtually indistinguishable from tubercular granulomas or other chronic granulomas. It is well defined in nature with T1-hypointensity, T2 hyperintense center with hypointense wall, and showing mild to moderate ring-like contrast enhancement. Presence of a concomitant sinonasal disease points toward fungal etiology. Dural enhancement is generally seen in lesions adjacent to involved sinuses ( Fig. 3 ).
To identify all relevant MRI features in intracerebral aspergillosis, MRI protocol should include DWI and contrast-enhanced T1-weighted images and T2-weighted sequences. For an early detection of hemorrhage, T2∗-weighted sequences play an important role in clinical practice.
On MR spectroscopy fungal abscesses show cytosolic amino acids, lipids, and lactate, which are similar to that of pyogenic abscesses; however, the presence of multiple signals seen between 3.6 and 3.8 ppm because of the disaccharide trehalose is a distinguishable feature of fungal abscesses.
Cryptococcosis
Cryptococcus neoformans is an encapsulated yeast-like fungus found in mammal and bird feces, particularly in pigeon droppings. C neoformans is the most common opportunistic fungal infection that affects the CNS in HIV and other immunocompromised patients. However, in approximately 30% patients, no definite predisposing disease may be seen.
Infection is usually acquired by inhalation and then spreads hematogenously to the CNS. The CNS infection can be either meningeal or parenchymal. Infection usually starts as meningitis and is more pronounced at the base of the brain.
Parenchymal involvement is seen as cryptococcomas, dilated Virchow-Robin spaces, or enhancing cortical nodules. The most common parenchymal sites are the midbrain and the basal ganglia. As the infection spreads along the Virchow-Robin spaces, the perivascular spaces may dilate with mucoid gelatinous material that is produced by the capsule of the organism and looks like cysts. These cysts are also called “gelatinous pseudocysts.”
Imaging features
The imaging manifestations of cryptococcosis are varied and frequently minimal. The imaging findings may consist of meningoencephalitis, intraventricular or intraparenchymal cryptococcomas, gelatinous pseudocysts, or hydrocephalus ( Fig. 4 ). Communicating hydrocephalus occurs because of the acute meningeal exudates and also may occur late in the course of the infection because of meningeal adhesions. It is the most frequent finding of cryptococcal infection.
On CT, cryptococcal lesions may have high or low attenuation. MRIs demonstrate T1 and T2 prolongation. Enhancement varies, but it is more likely to occur in immunocompetent hosts, because they can build up an effective inflammatory response. Cryptococcal lesions do not have restriction of diffusion, which distinguishes them from pyogenic abscesses. Meningoencephalitis results in T2 hyperintensity within the region of involvement and meningeal enhancement may be seen ( Fig. 5 ). The cryptococcomas are more common in immunocompetent patients and these may show enhancement because of high immune response of the host. MR spectroscopy shows marked increase in lactate with decrease in N -acetyl aspartate, choline, and creatine.
Mucormycosis
Mucormycosis is an acute and often lethal opportunistic infection, typically affecting immunocompromised patients or those with diabetes. It is caused by one of the members of the mucoraceal family, including Absidia , Mucor , and Rhizopus . After infection of the nasal cavity and paranasal sinuses, the fungi cause a necrotizing vasculitis that extends rapidly into deep face, orbits, cranial cavity, and brain through skull base partitions and foramina.
In particular, the fungus has an affinity for the internal elastic lamina of the arteries, through which it can hematogenously disseminate to distant sites. This process of cerebral angioinvasion produces extensive endothelial damage that can lead to mycotic aneurysms, dissection, or thrombosis of the internal carotid artery with subsequent cerebral infarction.
Imaging features
Imaging modalities include CT and MRI. In early stage disease both CT and MRI may be normal because radiologic findings lag behind clinical progression. CT is useful in assessing the disease extent and typically shows mucosal thickening, opacification of the affected paranasal sinuses, and periorbital tissue destruction. CT is the optimal imaging modality to assess for bony destruction.
On MRI, hypointensity of the sinuses may be present on T1- and T2-weighted images. Intracranially, high T2-weighted imaging signal in the basal portions of the frontal and temporal lobe with mild mass effect is seen; this likely represents a combination of inflammation and infarction secondary to vascular invasion.
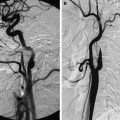
MRI is superior in visualizing and evaluating intracranial and intradural extent of the disease. Thrombosis of the cavernous portion of the internal carotid artery is well detected on MR angiography ( Fig. 6 ). Less commonly a hematogenous pattern of spread leading to central infarcts or granulomas may be seen in the brain secondary to Mucor infection ( Fig. 7 ).