Central nervous system (CNS) tuberculosis is frequently encountered in tropical countries. Imaging plays an important role in its recognition and in its differentiation from other similar conditions. Specific magnetic resonance techniques, such as magnetization transfer imaging, proton magnetic resonance spectroscopy, diffusion, and perfusion imaging are useful in its characterization and management. This article reviews the various forms of CNS tuberculosis, including its complications and imaging features.
Approximately one-third of the world’s population is currently infected with tuberculous bacillus, of which approximately 5% to 10% become sick or infectious at some time during their life. People with human immunodeficiency virus (HIV) are more likely to develop tuberculosis (TB). The World Health Organization (WHO) estimates that there were 9.4 million new cases in 2009, including 1.1 million cases among people with HIV. Approximately 1.7 million people died of TB, including 380,000 people with HIV. Most cases were in the south-east Asian, African, and western Pacific regions (35%, 30%, and 13%, respectively). In 2009, the estimated per capita TB incidence was stable or decreasing in all 6 WHO regions. However, the slow decline in incidence rates per capita is offset by population growth. Consequently, the number of new cases arising each year is still increasing globally in the WHO regions of Africa, the eastern Mediterranean, and south-east Asia.
- •
Central nervous system (CNS) tuberculosis is a major cause of sickness and death, especially in developing countries, and is increasing in developed countries because of the emergence of acquired immunodeficiency syndrome (AIDS).
- •
Isolation of Mycobacterium tuberculosis for the definitive diagnosis is possible only in a few patients. Culture has a low yield and needs 6 to 8 weeks.
- •
In tuberculous meningitis (TBM), precontrast magnetization transfer (MT) T1 imaging shows abnormal meninges with low MT ratio and is characteristic of the disease.
- •
Tuberculomas have solid and/or liquid caseation on noncontrast MT T1 images, a bright rim around T2 hypointensity is a characteristic feature of tuberculoma; the T2 hypointense rim appears bright in tuberculous abscess.
- •
When liquefaction of the caseation occurs within tuberculoma as well as abscess, it shows restriction on diffusion-weighted (DW) imaging with low apparent diffusion coefficient (ADC).
- •
Advanced imaging methods such as perfusion imaging and diffusion tensor imaging (DTI) may be of value in objective assessment of therapy in tuberculoma.
Neurotuberculosis constitutes 1% of all tuberculosis and 10% to 15% of the extrapulmonary tuberculosis cases, most (>40%) of which are children. Central nervous system (CNS) tuberculosis also accounts for 1.5% to 3.2% of all tuberculosis-related deaths. CNS tuberculosis remains common and, despite the availability of effective antituberculous therapy, continues to cause significant morbidity and mortality. TBM constitutes 70% to 80% of all patients with neurotuberculosis. CNS tuberculosis is a prominent cause of sickness and death in developing countries. In developed countries, there has been an increase in the number of CNS TB cases, possibly related to the pandemic of AIDS. M tuberculosis is responsible for almost all cases of tubercular infection in CNS. Tubercular bacilli initiate a granulomatous inflammatory reaction involving different tissue types of the CNS such as meninges, brain, spinal cord, and covering bones. The CNS manifestation is in a variety of forms, such as TBM and its complications, focal cerebritis, tuberculoma, and tubercular abscess. Spinal cord infection is less common and causes either arachnoiditis and/or intramedullary tuberculomas.
Early diagnosis and treatment of CNS tuberculosis is necessary to reduce the morbidity and mortality. Noninvasive imaging modalities such as computed tomography (CT) and magnetic resonance (MR) imaging are routinely used in the diagnosis of CNS TB; however, MR imaging is preferred because it offers greater inherent sensitivity and specificity than CT. This article reviews the various forms of CNS tuberculosis, including its complications and imaging features.
Pathophysiology
TBM
Tuberculosis is most often a primary infection in children and a postprimary infection in adults. CNS tuberculosis transpires hematogenously from a distant active site such as lung, bone, lymph nodes, or gastrointestinal or genitourinary tract. In the brain, the bacilli lodge in the cortical and subcortical regions and/or meninges, which are richly vascularized. Rarely, there is a direct spread from adjacent infected paranasal sinuses or mastoid air cells. Infection typically begins in a subpial or subependymal cortical location called the Rich focus. The site of this focus determines the type of CNS involvement. Initially, a nonspecific inflammatory reaction, tuberculous cerebritis, develops. Once sensitized, the inflammatory response results in a granuloma. This granuloma may erode into the subarachnoid space and cerebrospinal fluid (CSF), causing basal leptomeningitis. Subsequently, this leads to communicating hydrocephalus, and occasionally obstructive hydrocephalus caused by obstruction of the foramina of Luschka and Magendie. Vasculitis involving the lenticulostriate and thalamoperforating arteries may occur. The adventitial layer of these vessels develop changes similar to those of the adjacent tuberculous exudates followed by the intima, which may eventually be involved or be eroded by a fibrinoid-hyaline degeneration. In later stages, the lumen of the vessel may become completely occluded by reactive subendothelial cellular proliferation and cause small infarcts in the deep gray matter nuclei and deep white matter.
Tuberculoma
The initial lesion, a tubercle, consists of a central area of incipient or frank necrosis surrounded by inflammatory cells, lymphocytes, epithelioid and Langerhans giant cells, with an encircling rich vascular zone. These lesions begin as a conglomerate of microgranulomata that join to form a noncaseating tuberculoma. Following the initial infection, most such lesions resolve and reactivation or further evolution of these lesions manifests as caseation within the center of this tuberculoma. Rarely, the lesion may continue to grow by successive addition of layers of granulation tissue and form growth rings.
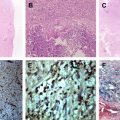
Subsequently, central caseous necrosis develops in most cases, which is initially solid surrounded by a capsule comprising collagenous tissue, epithelioid cells, multinucleated giant cells, and mononuclear inflammatory cells. The central core of solid caseation consists of a cheesy material high in lipid contents, with macrophage infiltration, regional fibrosis/gliosis, macrophage by-products (free radicals), and perilesional cellular infiltrates. A few bacilli may be present in the center. The caseation then usually liquefies, beginning from the center. The capsule consists of granulation tissue and compressed glial tissue. If abscess formation occurs, this shows central cavitation with chronic inflammatory reaction with fibrosis in the wall and the aspirate from the pus stains positive for acid-fast bacilli. All these lesions are usually accompanied by perilesional edema with some proliferation of astrocytes in the surrounding brain parenchyma.
Spine
M tuberculosis infection in the spine can involve any compartment in the spinal region: vertebrae, intervertebral disk, spinal cord, and its meninges. Meningeal involvement causes spinal meningitis and spinal arachnoiditis. The pathophysiology of spinal meningitis is the same as described earlier in TBM: during primary infection a submeningeal tubercle forms that ruptures into the subarachnoid space. It causes granulomatous inflammation, areas of caseation, and tubercles, with development of fibrous tissue in chronic or treated cases.
Clinical features
Children and older persons are more vulnerable to develop CNS tuberculosis because their immune systems are less robust. TBM is also common in immune-suppressed patients (those with HIV or diabetes, and patients taking steroids or cytotoxic drugs). The onset of TBM is insidious and may be characterized by persistent low-grade fever, malaise, headache, and confusion. Typical clinical features of TBM include fever with nausea, vomiting, headache, neck stiffness, and photophobia. Cranial nerve palsies, especially of third, fourth, and sixth nerves, may also be present. The intracranial tuberculomas manifest with features of a space-occupying lesion of the brain and the patients can present with features of increased intracranial tension, focal or generalized seizures, and focal neurologic deficit. Isolation of M tuberculosis for the definitive diagnosis from the tissue on smear or culture is possible only in a few patients. Culture takes 6 to 8 weeks for the result and has a low yield. Thus the index of suspicion is mostly indirect; that is, concomitant tubercular involvement elsewhere (only 10% of cases may show disease elsewhere in the body), malaise, low-grade fever, loss of weight, positive tuberculin test, increased sedimentation rate, history of contact, and so forth.
CSF analysis in TBM normally shows a lymphocytic pleocytosis, increased CSF protein level, and decreased CSF sugar concentration. CSF culture for acid-fast bacilli and CSF polymerase chain reaction (PCR) examination are confirmatory tests for the diagnosis of TBM. The sensitivity of CSF culture for detection of acid-fast bacilli has been reported to be approximately 50%. CSF PCR examination is a new technique, and is more sensitive than the combination of microscopic examination and culture for M tuberculosis .
Clinical features
Children and older persons are more vulnerable to develop CNS tuberculosis because their immune systems are less robust. TBM is also common in immune-suppressed patients (those with HIV or diabetes, and patients taking steroids or cytotoxic drugs). The onset of TBM is insidious and may be characterized by persistent low-grade fever, malaise, headache, and confusion. Typical clinical features of TBM include fever with nausea, vomiting, headache, neck stiffness, and photophobia. Cranial nerve palsies, especially of third, fourth, and sixth nerves, may also be present. The intracranial tuberculomas manifest with features of a space-occupying lesion of the brain and the patients can present with features of increased intracranial tension, focal or generalized seizures, and focal neurologic deficit. Isolation of M tuberculosis for the definitive diagnosis from the tissue on smear or culture is possible only in a few patients. Culture takes 6 to 8 weeks for the result and has a low yield. Thus the index of suspicion is mostly indirect; that is, concomitant tubercular involvement elsewhere (only 10% of cases may show disease elsewhere in the body), malaise, low-grade fever, loss of weight, positive tuberculin test, increased sedimentation rate, history of contact, and so forth.
CSF analysis in TBM normally shows a lymphocytic pleocytosis, increased CSF protein level, and decreased CSF sugar concentration. CSF culture for acid-fast bacilli and CSF polymerase chain reaction (PCR) examination are confirmatory tests for the diagnosis of TBM. The sensitivity of CSF culture for detection of acid-fast bacilli has been reported to be approximately 50%. CSF PCR examination is a new technique, and is more sensitive than the combination of microscopic examination and culture for M tuberculosis .
Imaging
Imaging features of tubercular infections of the CNS, which may involve the meninges, brain, spinal cord, bones covering the brain and spinal cord, are as follows.
Imaging Protocol
The MR imaging protocol for CNS tuberculosis includes T2, T1, fluid-attenuating inversion recovery (FLAIR), magnetization transfer (MT) T1, susceptibility-weighted, DW imaging, and postcontrast T1-weighted images. In addition, inclusion of 1 H MR spectroscopy for lesions more than 2 cm is helpful. The closest differential diagnosis of brain tuberculomas is neurocysticercosis (NCC) in the regions endemic for NCC. If lesions around 2 cm appear hyperintense on T2-weighted imaging and showing ring enhancement on postcontrast T1-weighted imaging, isotropic fast imaging excitation with steady state acquisition (FIESTA) should also be performed to show the scolex within a cyst, which is pathognomonic of NCC.
Cranial Tuberculosis
TBM
TBM is still a common problem in some parts of the world. The meninges are involved either by hematogenous seeding or by local spread from adjoining infected areas.
During the early stages of disease, conventional noncontrast MR imaging studies usually show little or no evidence of any meningeal abnormality. MT T1 imaging is considered superior to conventional spin-echo sequences for imaging the abnormal meninges, which are seen as hyperintense on precontrast T1-weighted MT images. As the disease progresses, mild shortening of T1 and T2 relaxation times may be seen compared with normal CSF. Postcontrast T1-weighted images show abnormal meningeal enhancement ( Figs. 1–3 ). The common sites are interpeduncular fossa, pontine cistern, perimesencephalic cistern, suprasellar cistern, and sylvian fissures, with occasional involvement of sulci over the convexities (see Fig. 1 ).
MT ratio (MTR) quantification helps in identifying the cause of chronic meningitis; low MTR suggests TBM. Ex vivo MR spectroscopy of the CSF shows lactate, acetate, and sugar signals along with cyclopropyl rings (−0.5 to +0.5 ppm) and phenolic glycolipids (7.1 and 7.4 ppm), which are not seen in pyogenic meningitis. The combination of ex vivo MR spectroscopy with MT MR imaging may be helpful in diagnosing TBM.
The secondary complications of TBM may develop as the disease progresses or even while the patient is on treatment. The sequelae associated with TBM are as follows.
Hydrocephalus
Hydrocephalus develops commonly as a result of blockage of the basal cisterns by the inflammatory exudates (communicating type) (see Fig. 2 ), or occasionally due to mass effect of a focal parenchymal lesion or entrapment of the ventricle by granulomatous ependymitis (obstructive type). Periventricular hyperintensity on T2-weighted or FLAIR images usually suggests seepage of the CSF fluid across the white matter. Atrophy of brain parenchyma may be a late sequela of chronic hydrocephalus. The choroid plexus may serve as an entry point for the pathogens into the CNS. Its involvement, choroid plexitis, presents as prominent contrast enhancement of the choroid plexus and is usually associated with ependymitis, ventriculitis, and meningitis.
Vasculitis
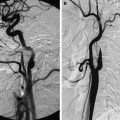
Vasculitis is another common complication seen at autopsy in cranial TBM involving small and medium-sized vessels and causing ischemic cerebral infarction. Most of the infarcts are in the basal ganglia and internal capsule regions because of the involvement of the lenticulostriate arteries; however, involvement of the large vascular territory such as the middle cerebral artery may also be encountered. MR angiography may help in the detection of vascular involvement (see Fig. 3 ). Intracerebral inflammatory aneurysms may also be seen in CNS tuberculosis. MR angiography can reveal this rare vascular complication. DW imaging helps in early detection of infarcts. Vascular complications are usually seen following initiation of specific therapy, possibly due to the healing and fibrosis of meninges resulting in the occlusion of embedded vasculature.
Focal or diffuse pachymeningitis
CNS TB may involve the dura mater, causing pachymeningitis, which may occur either in isolation or with pial or parenchymal disease. Pachymeningitis may present as focal or diffuse involvement of the dura mater and occur secondary to hematogenous spread. Thickened dura mater may be evident on precontrast studies but is detected usually as abnormal enhancement on postcontrast images. However, the appearance of focal and diffuse pachymeningitis on MR imaging is nonspecific and may be seen in a large number of inflammatory and noninflammatory conditions.
Cranial nerve neuropathy
Clinical involvement of the cranial nerves is seen in 17.4% to 40% of patients with TBM caused by vascular compromise resulting in ischemia of the nerve or caused by entrapment of the nerves by the exudates.
Intracranial tuberculoma
Brain tuberculoma is a space-occupying mass of granulomatous tissue that is encountered more frequently in developing countries and is responsible for high morbidity and mortality. The incidence of tuberculoma is higher in the developing world (15%–50% of all intracranial lesions), compared with developed countries where the incidence is about 0.2% of all biopsied brain lesions. Early recognition and treatment of this condition on imaging plays an important role in patient management. Intracranial tuberculomas may be solitary or multiple and variable in size. These tuberculomas are found across all age groups; however, a predilection for children has been reported. The common sites include cerebral hemispheres, basal ganglia, cerebellum, and brainstem. Rarely, ventricular system and meninges are also involved.
Extra-axial tuberculomas may also occur, which may cause widening of the basal foramen or adjacent bone destruction. Tuberculoma in the hypophyseal region is rare and it is frequently associated with thickening of the pituitary stalk.
MR features
From the appearance on T2-weighted imaging, the intra-axial tuberculomas may be classified as:
- 1.
T2 hyperintense lesion
- 2.
T2 hypointense lesion
- 3.
T2 hyperintense center with peripheral hypointense rim
- 4.
Lesion with mixed/heterogeneous signal intensity.
T2 hyperintense lesion
Noncaseating tuberculomas, typically less than 1.5 cm in diameter, appear hyperintense on T2-weighted images, isointense to hypointense on T1, hyperintense on MT T1, and FLAIR, and show nodular or ring enhancement on postcontrast studies. On DW imaging, these lesions may show hyperintensity with low ADC. These tuberculomas may be part of miliary tuberculosis or TBM ( Fig. 4 ). This appearance may resemble metastases, lymphomas, demyelinating plaques, and other infective granulomas. The presence of a bright rim on noncontrast MT T1 along with low MTR may help in its differentiation from other lesions.
T2 hypointense lesion
Tuberculomas with solid caseation are usually isointense to hypointense on both T2-weighted and T1-weighted images. These lesions are surrounded by a rim of variable thickness that may appear hyperintense on T1-weighted and T2-weighted images. On DW images, there is no restriction seen in the solid caseation of the tuberculoma with a high ADC. On noncontrast MT T1 images, this rim shows hyperintensity, a characteristic feature seen in tuberculomas, and the solid caseation remains hypointense ( Figs. 5–7 ). The rim comprises inflammatory cells, some of which may contain fragmented portions of the lipid-rich cell wall of M tuberculosis . Lipids are known to have no MT effect; the rim has a lower MT ratio than the core and appears bright on MT. Biochemically, the core contains necrotic tissue and macromolecules that are responsible for the higher MT ratio compared with the rim. The rim shows enhancement on postcontrast T1 images, whereas solid caseation does not enhance. The solid caseation contains cheesy material high in lipid contents, with macrophage infiltration, regional fibrosis/gliosis, and macrophage by-products (free radicals), components that are possibly responsible for the hypointensity seen on T2-weighted images. These MT T1 visible constituents of tuberculoma closely match the histologic appearances. The MT ratio of the core has been shown to be much lower than similar-appearing cysticerci lesions. In vivo MR spectroscopy of these tuberculomas reveals the presence of lipid peaks. The presence of serine at 3.7 to 3.9 ppm on ex vivo/in vitro MR spectroscopy of the tuberculoma sample suggest the presence of M tuberculosis because serine is found in abundance in the wall of this bacterium. T2 hypointensity is seen in some lymphomas, glioblastoma, metastases from colonic carcinoma or melanoma, fungal granulomas, or cysticerci lesions. Hemorrhage, hemorrhagic tumor, occult cerebrovascular malformation, and calcification may also appear as T2 hypointense lesions. Susceptibility-weighted sequences such as SWAN may differentiate hemorrhage and calcification from other T2 hypointense lesions.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree