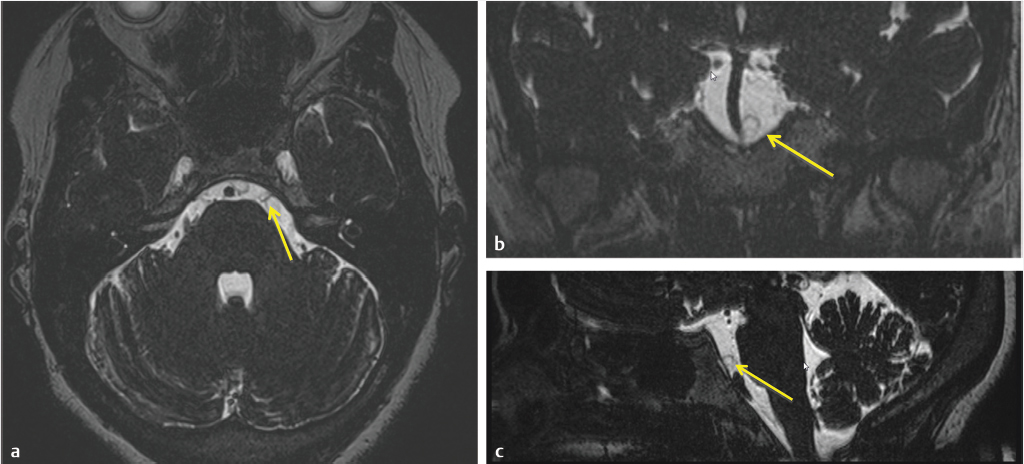
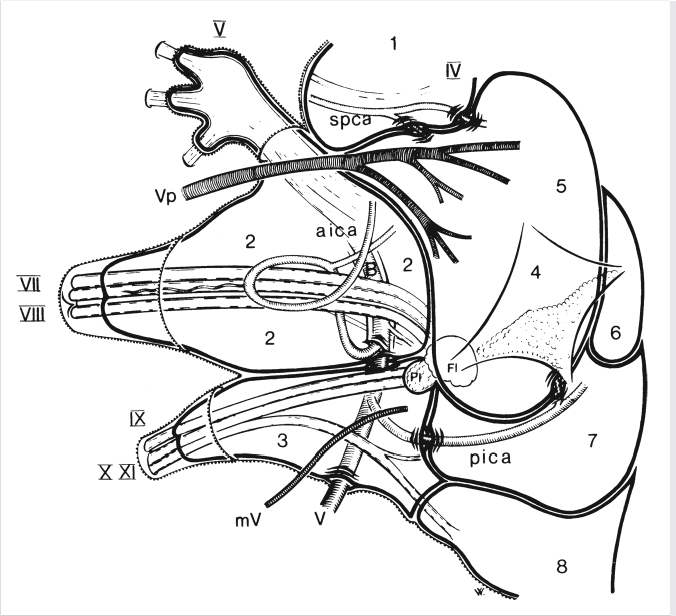
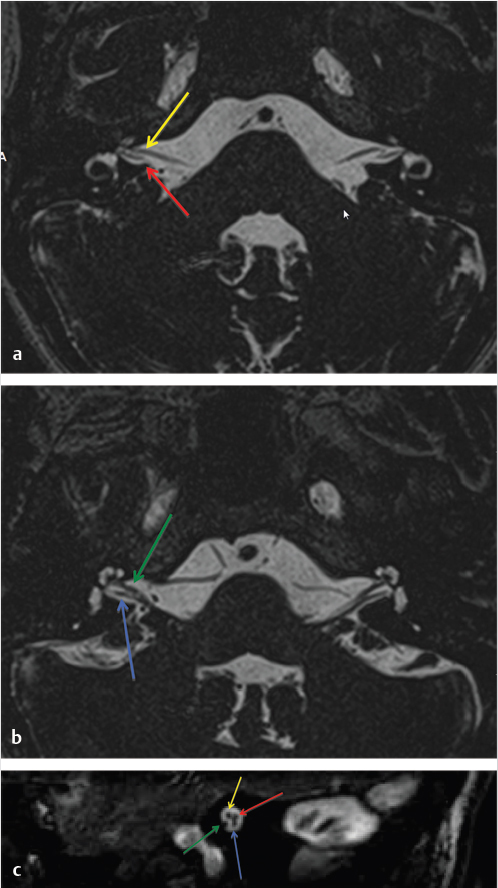
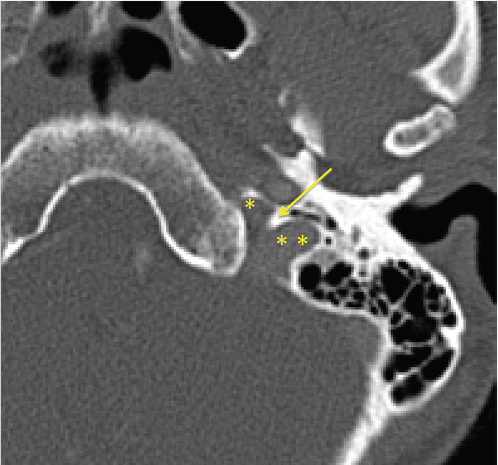
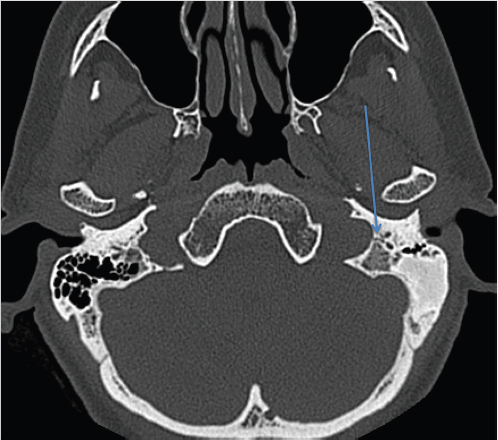
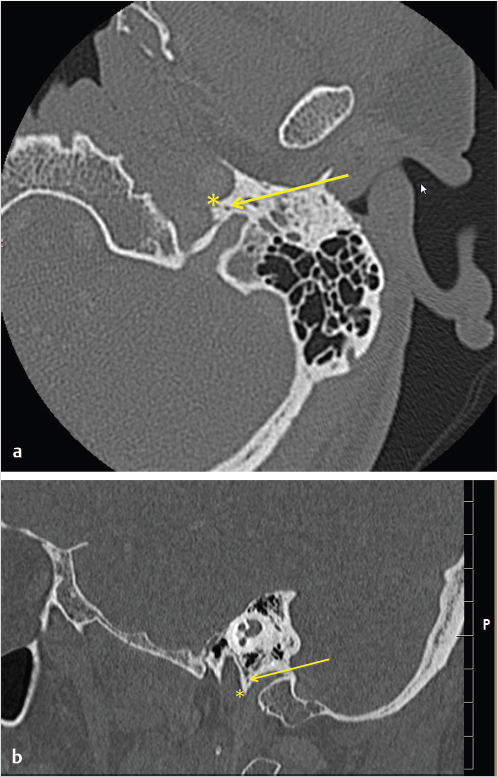
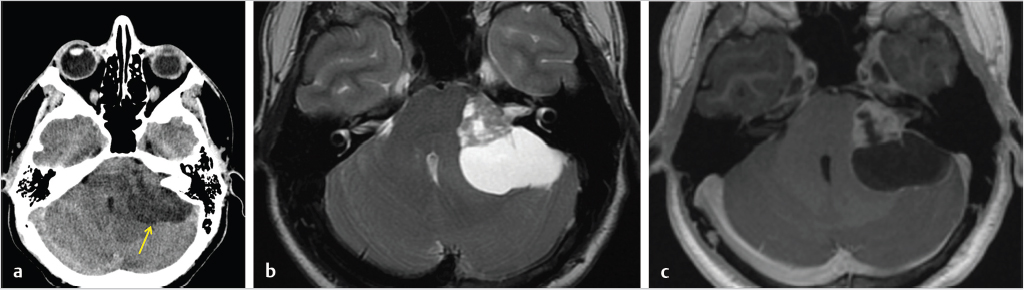
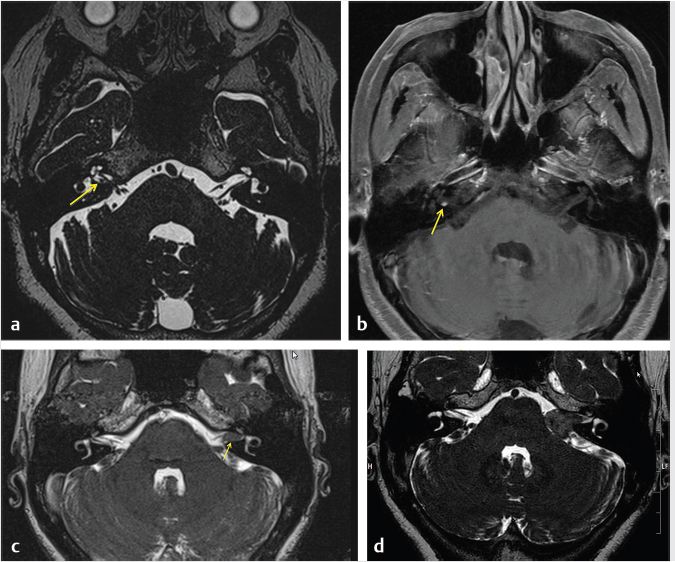
3 Cerebellopontine Angle and Jugular Fossa The cerebellopontine angle (CPA) cisterns are paired lateral infratentorial cerebrospinal fluid (CSF)-filled spaces bound by the pons, cerebellum, and petrous temporal bones. On axial images, they are triangular in shape. Although they communicate with other cisterns, they are also compartmentalized by porous trabeculated walls ( The literature of the CPA cistern can be confusing because it has been subdivided by some researchers into a superior compartment (this corresponds to what is generally regarded by most as the “CPA cistern”) and an inferior compartment (which is called the “lateral cerebellomedullary cistern”). The contents are shown in The boundaries of the CPA cistern are as follows: • Posteriorly: The posterior quadrangular and superior semilunar lobulus of the anterior cerebellar hemisphere. • Posteromedially: The pontomedullary sulcus, the flocculus of the cerebellum. • Medially: The lateral portion of pons. It is continuous with the prepontine cistern superiorly and premedullary cistern inferiorly. • Superiorly: The ambient cistern just below the tentorial hiatus. The CPA roof is limited by the tentorium and its attachment to the petrous bone. • Inferiorly: The lateral cerebellomedullary cistern. The lateral outlet of the fourth ventricle, the foramen of Luschka, marks the boundary with the lateral cerebellomedullary cistern. • Laterally: The posterior petrous segment of the temporal bone, internal auditory meatus, and Meckel’s cave. The CPA cistern extends into the internal auditory canal (IAC) around the cranial nerves (CNs) VII and VIII. The normal orientation of the CNs at the IAC porus acusticus is as follows: The facial nerve is anterosuperior, the cochlear nerve is anteroinferior, the superior vestibular nerve is posterosuperior, and the inferior vestibular nerve is anteroinferior. “Seven-up, Coke down” is an easy way to remember their location ( The jugular bulb is the junction between the lower sigmoid sinus and the internal jugular vein (IJV). It is situated between the occipital bone and the mastoid portion of the temporal bone. The lateral bony wall of the intraosseous jugular bulb is adjacent to the hypotympanum and, when dehiscent, can cause pulsatile tinnitus. An alternate name for the jugular bulb is the jugular fossa (JF). When the top of the jugular bulb extends cranial to the plane of the lateral semicircular canal, it is referred to as a high-riding jugular bulb. The right jugular bulb is larger than the left bulb in approximately 80% of people, as the primary drainage of blood from the brain is on the right side. The JF courses anteriorly, laterally, and inferiorly and is divided by a fibrous or a bony septum into two compartments, the larger posterolateral pars vascularis and the smaller anteromedial pars nervosa ( Fig. 3.1 High-resolution T2-weighted sequence in the posterior fossa in axial (a), coronal (b), and sagittal (c) sequences. There is demonstration of normal membrane or porous trabeculations (arrows) within the prepontine cistern. These can also be seen in the cerebellopontine angle cistern. The pars vascularis contains the jugular bulb, part of the sigmoid sinus, CN 11, and CN 10 with its auricular branch (nerve of Arnold). The latter can be seen on thin section computed tomography (CT) traversing through the mastoid canaliculus on the lateral wall of the JF adjacent to the mastoid segment of the CN 7 ( The four most common CPA cistern lesions are the vestibular schwannoma (VS), meningioma, epidermoid cyst, and nonvestibular posterior fossa schwannoma. Together, they account for 75 to 98% of CPA cistern mass lesions.5 The VS is a benign slow-growing primary nerve sheath neoplasm that arises from the Schwann cells which wrap around CN 8 in the IAC. This tumor has no malignant potential. VS involves the superior vestibular division of CN 8 far more commonly than the cochlear division, and hence the name VS, which was officially recommended by the National Institutes of Health Consensus Developmental Panel in 1991.6,7 Less favored terms are acoustic schwannoma or neurinoma. VS accounts for 8 to 10% of all intracranial neoplasms and it is the most common CPA cistern mass accounting for 85 to 90% of the cases. It is also the second most common extra-axial mass in adults. Sporadic VS is the most commonly (> 90%) found lesion in patients with unilateral sensorineural hearing loss (SNHL). The incidence of sporadic VS is 0.2% across all imaging scans in asymptomatic patients. Mutations of the neurofibromatosis 2 (NF2) tumor suppressor gene is present in 60% of sporadic VS. There is a female predominance of up to 2:1, especially for larger and more vascular tumors and pregnancy can potentially accelerate the clinical course. It has also been reported that purely intracanalicular tumors are more common in males. The most common age of presentation is in the fourth to fifth decades of life and VSs more commonly affect Caucasians. When VS is present in young adults or children, or is associated with other lesions, a careful evaluation for other features of neurofibromatosis should be performed. Fig. 3.4 High-resolution unenhanced axial CT image at the level of the jugular foramen. The jugular fossa is divided by a bony septum (arrow) into two compartments, the larger posterolateral pars vascularis (**) and the smaller anteromedially situated pars nervosa (*). Fig. 3.5 Axial CT image shows the mastoid canaliculus (arrow) connecting the jugular fossa to the facial nerve canal. The vestibular nerve has a transitional zone from oligodendrocytes to Schwann cells at the porus acusticus. Therefore, most VSs arise inside the IAC, or near its opening, but they can then secondarily grow into the CPA cistern. Small VSs are usually entirely contained within the IAC. The most common presenting symptom is a slowly progressive SNHL mainly affecting the perception of high-frequency sounds. Vertigo, tinnitus, and balance issues are uncommon (< 10%). Other symptoms can arise secondary to mass effect on the facial and trigeminal nerves, the brain stem, cerebellum, or fourth ventricle (obstructive hydrocephalus). Rarely, large lesions can present with subarachnoid hemorrhage. Brain stem–evoked response audiometry (BERA) is considered a sensitive preimaging test for diagnosis.3,5,8,9 Fig. 3.7 (a) Contrast CT demonstrates a heterogeneous mass in the left cerebellopontine angle (CPA; arrow) that is causing mass effect on the adjacent brain parenchyma. (b) Axial T2-weighted MRI demonstrates a mixed solid and cystic left cerebellopontine angle mass with extension into the internal auditory canal (IAC). (c) Axial T1-weighted postgadolinium image demonstrates enhancement of the solid component and extension into the left IAC. There is no consensus on a size grading scale for VS, but some have adopted the following10: • Small VS: Limited to the IAC with less than 1 cm extension into the CPA cistern. • Medium VS: 1 to 2 cm sized cisternal component. • Large VS: 2 to 4 cm sized cisternal component. • Giant VS: Greater than 4 cm sized cisternal component. Small VS or those that are completely intracanalicular can easily be missed on routine CT and magnetic resonance imaging (MRI) of the brain. When VS is suspected, a high-resolution temporal bone CT or, more preferably, a MRI that includes focused high-resolution imaging of the IAC and posterior fossa should be performed preferably with contrast enhancement. The imaging features of VS reflect the presence of the two types of cellular regions present within schwannomas. Antoni A regions are the highly cellular dense regions which forms palisades histologically. This is reflected on imaging as a solid and homogenously enhancing tumor. The Antoni B regions are hypocellular with cells separated in a reticulated myxoid matrix. On imaging, this gives rise to areas of cystic change in the VS. CT features of VS are variable. Small intracanalicular lesions may not be visible unless they expand or remodel the bony margins of the IAC. Larger lesions may be visible once they extend into the CPA cistern and cause mass effect upon the adjacent brain ( Erosion and enlargement/flaring of the porous acusticus can be seen to occur in 70 to 90% of VS with or without a CPA cisternal soft-tissue component.12 MRI is generally the best imaging test for the assessment of VS. A high-resolution T2-weighted sequence can detect lesions as small as 0.06 cm.13 Small VSs appear as a round or funnel-shaped mass centered in the long axis of the IAC. Larger VSs are seen as a well-marginated spherical, ovoid, or lobulated CPA cistern mass with a convex medial margin and extension into a widened porous ( Fig. 3.8 (a) Axial high-resolution T2-weighted MRI shows a 1- to 2-mm rounded lesion (arrow) in the right internal auditory canal (IAC) compatible with a small VS. (b) Fat-saturated axial T1-weighted postgadolinium demonstrates nodular enhancement of the lesion (arrow). (c) High-resolution MRI T2-weighted image of a larger 8-mm elongated lesion filling left IAC in keeping with a VS (arrow). (d) Axial high-resolution MRI T2-weighted image sequence of the same patient as in (c). This scan was taken 6 years later showing interval growth and the extension of the lesion into the cerebellopontine angle cistern with secondary mass effect on the left pons/middle cerebellar peduncle. Fig. 3.9 (a) Axial T2-weighted image of a very large VS (arrow) causing mass effect upon the adjacent cerebellum. There is marked compression of the fourth ventricle. The mass also forms acute angles to the posterior cisternal face of the petrous bone. (b) Axial image at a level more superiorly demonstrates secondary obstructive hydrocephalous as a result of the fourth ventricular compression. On T1-weighted images, VSs are isointense to gray matter. On T2-weighted images, VSs are usually hyperintense. An arachnoid cyst or trapped CSF can be associated with 0.5% of cases. A potential explanation for this is the trabeculae in the CPA cistern that may thicken and entrap CSF or accumulate hypertonic fluid rich in protein that is excreted from the surface of the tumor.2,14 Use of contrast enhancement on MRI can help the detection of VS approach 100% sensitivity.15 This is best accomplished with the use of T1-weighted, fat-suppressed sequences. Small lesions will homogeneously enhance, while larger VSs appear heterogeneous, and demonstrate degenerative changes with cystic regions (15%) and occasional hemorrhage (0.5%), thrombosis, and rarely fatty degeneration. With larger VS, it is important to assess the degree of mass effect on the brain stem and cerebellar peduncle, the presence of perilesional edema, and the development of obstructive hydrocephalus secondary to compression on the fourth ventricle ( There are certain MRI features that are important to note on follow-up studies as they may predict a higher risk for hearing loss: • A growth rate more than 2.5 mm/year is associated with a doubling of the risk of hearing loss.16 • Initial size has no bearing on the development of hearing loss. Size does not correlate with symptomatology and does not predict growth rate. • The presence of blood or fibrosis in the tumor may lead to vestibulocochlear damage and hearing loss. This is best evaluated on a T2* gradient sequence which will show marked reduction in signal intensity or “blooming.”16 • Impaction of tumor at the cochlear aperture.8 Tumors that fill the IAC have a worse prognosis for hearing preservation with surgery or stereotactic radiosurgery (SRS)/radiotherapy. Radiation therapy is one potential treatment option for VS. In a series of 86 patients by Nakamura,17 three patterns of contrast change were observed: 1. Transient loss of contrast, 84%. 2. Continuous increase in enhancement, 5%. 3. No change in the pattern of enhancement, 11%. There was no significant correlation between change in tumor volume and the degree of enhancement. An increase in T2 signal intensity in the adjacent brain stem and cerebellum was noted, reflecting the presence of edema. No correlation between radiation dose, tumor size, and the T2 signal changes was found. In another series of 89 patients by Norén et al,18 with 12 months of follow-up after radiosurgery, size changes were noted as follows; 73% remained stable, 22% shrunk, and 3% grew. Seventy-nine percent of patients lost their central enhancement 5 to 15 months post–radiation therapy and some regained central enhancement after a period of time. Enhancement in the adjacent parenchyma was also noted to occur. Nine percent had an increase in T2 signal in the adjacent parenchyma which did not correspond with focal neurological deficits. Delayed trigeminal enhancement was also noted. It should also be noted that for some partially resected or radiated VSs, such procedures can lead to a shedding of proteins from the tumor, which can result in communicating hydrocephalus in about 4% of cases. For follow-up, an annual CT or MR is often performed to monitor tumor growth. There is no relation between the initial VS size and subsequent growth rate. If there is a family history of NF2, screening with MRI of family members is recommended.2,3,5,8,9 Some important findings to assess on imaging include the following: • Size—especially, the volume of tumor. • Location—intracanalicular, CPA cistern, extension to tentorial hiatus, the jugular foramen or beyond. • Local mass effect—cerebellar, brain stem, edema, CNs, arteries, and veins. • Location of the jugular bulb. • Dominance of the transverse sigmoid sinuses. • Presence or absence of hydrocephalus. • Degree on tumor extension into the IAC. • Presence of enhancement along the facial nerve canal or the geniculate ganglion as this would suggest the presence of a facial nerve schwannoma rather than a VS. The size of the CPA component of VS is especially important as it will affect the method of treatment—namely, surgery versus radiation treatment. Surgery is usually preferred if the tumor is larger than 2.5 to 3.0 cm within the CPA, or if there is compression on the brainstem, brainstem edema, or obstructive hydrocephalus. Lesions that do not extend to the distal end of the IAC have a better prognosis for hearing preservation at surgery. This is primarily because there is a better chance that the blood supply, which is end-organ arterial (labyrinthine and cochlear arteries), can be preserved during surgery. Also, tumors that do not extend to the very distal end of the IAC will help limit the degree of dissection required for tumor exposure and removal. Before deciding upon a translabyrinthine versus retrosigmoid (suboccipital) approach, it is important to know the status of the sigmoid sinus and jugular bulb. Surgeons want an unencumbered and thus easier route of access to the region. The decision on preferred surgical approach is more often surgeon related than related to patient or tumor factors. However, if the jugular bulb is particularly high and the sigmoid on the ipsilateral side is dominant, this may limit the transtemporal approaches and favor a retrosigmoid route. On the other hand, larger lesions may also require more bone exposure to access the superior and inferior extensions of the lesion, something that is less limited via the retrosigmoid access. Seventy-five percent of VSs grow slowly, 10% grow rapidly (≥ 1 cm/year), and 15% grow very slowly. If left untreated, hearing loss followed by symptoms related to local mass effect will progress slowly over time. If hydrocephalus or intratumoral hemorrhage occurs, clinical deterioration can be rapid. Sudden irreversible, hearing loss can occur even in patients with small tumors. Patients with NF2 typically have worse outcomes. Treatment options include monitored observation, surgery, stereotactic single fraction radiosurgery (SRS), and fractionated stereotactic radiotherapy (SRT). The size of the lesion, age of the patient, comorbidities, and whether hearing is considered serviceable are the main patient-related factors affecting the choice of treatment. In a study by Hajioff et al19 that followed 72 patients for a minimum of 10-year follow-up, it was noted that when a tumor reaches the CPA a growth rate of approximately 1 to 2 mm/year in cross-sectional diameter was observed. Within the IAC, growth rates are generally less at 0.5 mm/year. Tumor growth rate in a subset of 19 patients with intracanalicular tumor was 0 mm/year. In the group with CPA extension less than 2 cm, the growth rate was 1 to 2 mm/year. About 15% of patients had some regression in the size of their tumor over this time frame. Most experts agree that watching a tumor until it reaches a size of approximately 2 cm within the CPA is reasonable. Once greater than 2 cm, some active treatment option could be considered (either microsurgical removal or SRS). However, others suggest intervention when growth is observed on at least on two serial scans. Maximal tumor dimension is an important consideration for surgery, given that the incidence of facial nerve paralysis at surgery increases from 5%, if the tumor is less than 2 cm, to 10 to 15% if larger than 2 cm but still less than 3 cm if total tumor removal is performed. A hybrid approach is increasingly being practiced for large tumors needing surgery. The surgical aim is to debulk the tumor and decompress the brainstem and brain parenchyma with a planned tumor residual being left in vicinity of the facial nerve. This residual can then be observed and/or treated upfront postoperatively with radiation. The aim of this approach is to minimize the chance of facial nerve damage that can be debilitating to patients. For those patients in whom surgery is being contemplated, the degree of hearing loss and the full extent of VS are important factors. If hearing preservation is of the utmost importance, a middle cranial fossa (only for intracanalicular tumors) or retrosigmoid/suboccipital approach is favored. The middle cranial fossa approach is best for intracanalicular VS situated more laterally in the IAC. A retrosigmoid approach is considered when there is a significant cisternal or medial IAC component. The translabyrinthine approach is used when there is already poor hearing or the mass is considered so large that hearing sacrifice is unavoidable. Specific to radiosurgery, positive predictors for hearing and facial nerve preservation post-SRS include tumor size (< 5 cm3), younger than 60 years, radiation dose less than 13 Gy, and dose to the cochlea (radiation plans that expose the cochlea to mean doses below 4–8 Gy have higher rates of hearing preservation). There is debate in the radiation treatment for patients with baseline hearing. The decision centers on whether hearing can be preserved maximally with fractionated SRT versus SRS due to the benefits of fractionation. This is because of the long-term late effects of radiation such as hearing damage, which are influenced by the dose per fraction. Currently, there are no conclusive data on this subject and future clinical trials will be needed to resolve these issues. Facial nerve schwannomas can involve any or multiple segments of the facial nerve. This is a rare tumor and can be associated with NF2. There is no gender predilection and the age of presentation is usually in the fifth decade of life. The geniculate ganglion is the most commonly affected site followed by the labyrinthine and then the CPA–IAC portion of the nerve. Involvement of the intraparotid segment is rare. Multisegment involvement is common. Fifty percent of the facial nerve schwannomas are asymptomatic. In the other 50% of the cases, the most common presentation is facial nerve paralysis which usually takes years to develop. This is likely related to the fact that schwannomas compress and do not invade the nerve. Symptoms will also depend on the segment involved. When the tumor is situated in the IAC or CPA, SNHL can be the presenting symptom due to secondary mass effect on the thinly myelinated sensory fibers of CN 8 which are more vulnerable to compression compared to the more thickly myelinated motor fibers of CN 7. Tumors involving the geniculate ganglion are often clinically silent. However, with continued growth, the mass can bulge into the middle cranial fossa and cause local mass effect upon the brain; it can also involve the greater superficial petrosal nerve resulting in a loss of lacrimation. These tumors may also cause hyperacusis due to involvement of the stapedial nerve and paralysis of the stapedius muscle. Tumors involving the tympanic segment of the nerve can give rise to conductive hearing loss due to interference with the ossicular chain. Facial schwannomas that arise in the mastoid segment are likely to cause facial palsy as the canal is a small, limited, and of fixed volume. Other symptoms include vertigo and hemifacial spasm. On occasion, large tumors involving the stylomastoid foramen can present as painless neck mass.2,5 As a facial nerve schwannoma can give rise to facial paralysis, it is important to differentiate it from idiopathic facial nerve palsy (Bell’s palsy). Patients with a Bell’s palsy typically have an acute onset and symptoms resolve within approximately 2 months. In those patients with history of progressive facial nerve paralysis beyond 3 weeks, absence of recovery after 6 months, or ipsilateral recurrence, a facial nerve schwannoma would be a consideration. Differentiation of other lesions affecting the facial nerve, such as autoimmune or viral diseases, is also necessary and this is most often investigated with imaging studies. A classic appearance on CT is an IAC mass with associated widening of the canal, thus mimicking a VS. However, careful assessment will show enlargement of the labyrinthine portion of the facial nerve as the schwannoma follows the natural course of the facial nerve through the fallopian canal. Enlargement of the geniculate ganglion may also be seen ( Facial schwannoma can also present as a posterior middle cranial fossa mass and the imaging clue would be the presence of contiguous extension toward the geniculate ganglion and or other segments of the facial nerve ( Care must be taken when assessing the facial nerve for enhancement as normal physiologic enhancement can be present along portions of the nerve. The normal facial nerve can demonstrate some enhancement on MRI in up to 76% of examinations, and in 69% of cases this can be asymmetric. This has been attributed to the presence of the normal circumneural facial arteriovenous plexus. Enhancement can be present in all segments except the cisternal, canalicular (IAC) portions and extracranial segment beyond the stylomastoid foramen.4,20 These latter portions normally do not enhance. Fig. 3.10 Axial high-resolution enhanced CT of the right temporal bones (a), in bone window (b), and in soft-tissue window (c) showing a widening of the right geniculate ganglion (yellow arrow; a) and labyrinthine (red arrow; b) portion of the facial nerve. (c) An enhancing mass widens the internal auditory canal and bulges into the cerebellopontine angle cistern. Fig. 3.11 Axial T2 high-resolution FIESTA sequence shows a large cerebellopontine angle mass that extends into the internal auditory canal. The enlargement of the geniculate ganglion (arrow) is compatible with this mass being a facial schwannoma. Some pathology that can mimic a facial nerve schwannoma includes a persistent stapedial artery which will enlarge the facial tympanic canal and an arachnoid diverticulum which may enlarge the geniculate ganglion. Critical imaging features to assess and report are the portion(s) of the facial nerve that are involved, whether the tumor extends into the IAC, and if there is any involvement of the inner ear. Watchful waiting is one approach as some facial nerve schwannomas may not grow. Intervention is considered once there is facial nerve paresis or other symptoms. Radiosurgery or fractionated radiotherapy will have a good chance of preserving facial nerve function. Since surgery requires facial nerve grafting, the best result obtainable would result in a House-Brackmann (HB) grade 3 score (moderate dysfunction, noticeable asymmetry at rest, and complete eye closure with effort). This degree of facial nerve outcome is often of significant concern to the patient. The surgical goal is complete tumor resection with preservation of hearing and restoration of facial nerve function by restoring the continuity of the nerve by either end-to-end anastomosis or cable grafting. Since surgery may actually worsen the symptoms,2,5,8 consideration must be given to all potential treatment modalities and in particular radiation therapy. If a patient develops an acute facial paralysis that does not improve over 6 months and the tumor is located within the horizontal or vertical segments of the facial nerve in the middle ear/mastoid segments, respectively, this may make surgical treatment more urgent. If, on clinical follow-up, the facial function deteriorates to a greater than House Brackmann grade 3/6 appearance, then surgery would more than likely be suggested. This is on the basis that a facial hypoglossal nerve anastomosis would at best provide a grade 3/6 appearance to facial function. The issue for the surgeon to consider is whether surgical decompression therapy would be a reasonable approach to alleviate pressure from the facial nerve and allow the tumor to expand. The main reasons to avoid surgical resection are the presence of significant medical comorbidities, or the fact that a resection will definitely result in permanent total facial nerve paralysis (HB grade 6/6). This latter outcome may cause many patients to elect to avoid surgery. SRS, and more often fractionated SRT (45–54 Gy over 5–6 weeks), is a treatment option for these patients. Although limited literature exists, it does not have the adverse event profile of permanent facial nerve palsy and this treatment can result in long-term local tumor control. Schwannomas can arise within the carotid sheath (CS) anywhere from the level of the skull base to above the aortic arch. CNs that can be involved include CNs 9, 10, and 11. Medial to the CS and in front of flexure muscles of the cervical spine are the sympathetic chain and superior sympathetic ganglion. The proximity to the CS structures warrants their inclusion in this discussion. Schwannomas usually arise in otherwise healthy individuals. The literature is confusing as some authors mention a male predominance,9 while others do not describe any such gender predilection.21 The affected age range can be broad ranging from 18 to 63 years. CS schwannomas can also be associated with NF2. The suprahyoid location of these tumors is much more common than an infrahyoid location. Seventeen percent to 25% of the lesions occur in the retrostyloid parapharyngeal space (PPS) with the most common nerves of origin in descending order being the vagus > glossopharyngeal > superior sympathetic chain. Small lesions are rarely symptomatic. Larger lesions typically present as a painless palpable neck mass or posterolateral pharyngeal wall mass at the level of the naso- or oropharynx. The infrahyoid lesions can present as an anterolateral neck mass. Large lesions can also have mass effect and result in dysphagia, IJV occlusion, Horner’s syndrome, sleep apnea, or a sore throat. Specific nerve palsies or deficits can occur and be a clue to the nerve of origin: • Vagal schwannomas: Hoarseness from vocal cord paralysis and pain radiating to the ear and eye, angle of mandible and tonsillar region. • Glossopharyngeal schwannomas: Paralysis of the ipsilateral stylopharyngeus muscle (this muscle helps elevate the larynx and expand the pharynx during swallowing) which can lead to some impairment of swallowing and speech, loss of taste in the posterior one-third of tongue, loss of temperature, touch and deep sensation of the base of tongue, Eustachian tube, pharynx and tonsil, and abnormal gag reflexes. • Accessory nerve schwannomas: Downward and lateral rotation of the scapula and shoulder droop from atrophy of the trapezius and sternocleidomastoid muscles with compensatory hypertrophy of the ipsilateral levator scapulae muscle in chronic cases. This is similar to the shoulder drop syndrome that can follow a radical neck dissection. Schwannomas arising within the CS appear as ellipsoid, smoothly marginated masses with either uniform enhancement or cystic areas. Imaging features are similar to all other schwannomas. When they arise within the JF, the margins of the surrounding bone are smoothly widened. The internal carotid artery (ICA) and IJV can be displaced by the schwannoma and the direction of carotid displacement may also help predict the likely nerve of origin. As the vagus nerve is posterior to the ICA within the CS, vagal schwannomas will displace the ICA anteriorly and usually medially while displacing the IJV posteriorly and laterally (splay common carotid artery or ICA away from IJV; Alternatively, since the superior sympathetic chain is situated medial to the CS, sympathetic chain schwannomas will typically displace all the vessels together anterolaterally. Such sympathetic schwannomas can also slip between the CS and pharyngeal wall and result in posterior displacement of the ICA ( Fig. 3.14 (a,b) Contrast-enhanced T1-weighted images demonstrate a left vagal schwannoma in the left carotid space that displaces the left internal carotid artery anteriorly and medially. The mass demonstrates intracranial extension through the jugular foramen. (c) Sagittal T1-weighted image shows the mass to be both cystic and solid with protrusion through the jugular foramen into the posterior fossa. Fig. 3.15 Contrast-enhanced axial CT (a) and axial T2-weighted (b) images of a sympathetic chain schwannoma. This well-marginated minimally enhancing lesion is displacing the carotid and jugular vessels posteriorly. A watch-and-wait approach is a consideration with CS schwannomas. Ultimately, experienced head and neck surgeons would resect a vagal schwannoma if there is 30 to 40% growth in size over 3 to 4 years, if there is compression on other CNs with progressive neurological symptoms, or if there is evidence of malignant transformation. Removal of a nerve sheath tumor generally causes a nerve palsy of the involved nerve if it has not occurred already from the tumor. The worst feared intraoperative complications are stroke related to injury of the carotid arteries and associated lower CN palsies that could result in significant comorbidities such as chronic aspiration and hoarseness. Fractionated SRT is a treatment option for these patients. Although limited literature is available, it does not have the adverse event profile of surgery and can yield long-term local tumor control. Paragangliomas are benign, slow growing, highly vascularized lesions originating from paraganglionic tissue of the extraadrenal nonchromaffin chemotactic cells which are distributed around the tympanic portion of the facial nerve, the jugular foramen, within the parapharyngeal portion of the vagus nerve and in the carotid body. These latter tumors are in an intimate relationship to the vagus nerve. Other names for these lesions include chemodectoma and glomus tumor. However, according to Glenner and Grimley,22 paraganglioma is the most appropriate name for these lesions. Paragangliomas have the potential for local invasion and can frequently invade the skull base with resulting intracranial extradural extension. These tumors rarely metastasize. Paragangliomas in the neck are named according to their site of origin: • Glomus jugulare: Situated at the level of the jugular bulb and skull base, arising from the tympanic branch of the glossopharyngeal nerve (nerve of Jacobson), or the auricular branch of the vagus nerve (nerve of Arnold). • Glomus tympanicum: Situated in the tympanic cavity and mastoid, along the course of Jacobson’s nerve. • Glomus jugulotympanicum: Those lesions span both the middle ear and jugular foramen. • Glomus vagale: From the intravagal paraganglia at or below the skull base level and primarily parapharyngeal in location. • Carotid body tumor: Arising from the carotid body at the carotid bifurcation. In this section, the glomus jugulare/jugulotympanicum and vagale tumors will be discussed. The incidence of paragangliomas is 1 per 1.3 million people per year and they are significantly more common in females with a male-to-female ratio of 3–4:1. Two-thirds of patients at the time of presentation are in their fourth to sixth decades of life. These lesions can be bilateral (2%) or multifocal (10%), with the most common association being a jugulare/jugulotympanicum paraganglioma and an ipsilateral carotid body tumor. Paragangliomas can also be familial, with a nearly 30% incidence of multiple lesions. The glomus jugulare/jugulotympanicum is the second most common tumor involving the temporal bone after VS. Ten percent of cases are associated with other entities such as medullary thyroid carcinoma, islet cell, or nonendocrine tumors of mesodermal origin, such as pulmonary chondroma and gastric leiomyosarcoma.23 The average time from symptom onset to diagnosis is 3 to 6 years. Glomus jugulare and tympanicum/jugulotympanicum are usually initially asymptomatic. When symptomatic, the presenting symptom depends on the location and extent of the tumor and the compression of surrounding neural structures. Very rarely, large paragangliomas can secrete norepinephrine or, less often, adrenocorticotropic hormone, serotonin, calcitonin, or dopamine. However, only 1 to 3% of these secreting paragangliomas present with clinical symptoms because the majority secrete low levels of hormones.2,23 Glomus jugulotympanicum can grow laterally to produce otologic symptoms (conductive hearing loss, pulsatile tinnitus, or retrotympanic mass) or can grow medially and extend further into the jugular foramen and cause jugular foramen syndrome (Vernet’s syndrome) consisting of CNs 9 to 11 deficits. The incidence of lower CN palsies is 35% with CN 10 palsy being the most common (61%)—CN 7 (45%), CN 11 (52%), CN 9 (48%)—and CN 12 being the least common. Compression of CN 12 will give rise to Collet-Sicard syndrome, which consists of unilateral CN 9, 10, 11, and 12 palsies. It is distinguished from Villaret’s syndrome by lack of sympathetic involvement.24,25 Involvement of CN 7 in the mastoid portion of the temporal bone can give rise to a facial palsy. Sympathetic chain involvement in the carotid canal can result in a Horner’s syndrome. Cavernous sinus extension will result in a cavernous sinus syndromes including ophthalmoplegia, diplopia (which could be acute or slowly progressive in onset and can be painful), and exophthalmos.26,27 Paragangliomas can also extend into the posterior fossa resulting in mass effect upon the brain stem/cerebellum and result in obstructive hydrocephalus. On CT, a glomus jugulare is usually seen as an enhancing mass centered in the JF that can cause irregular demineralization (moth-eaten appearance) and expansion of the margins of the JF and caroticojugular spine ( Fig. 3.16 Axial CT bone window (a) image of a right glomus jugulare tumor. There is expansion and permeative erosion of the jugular foramen. Axial (b) and coronal (c) soft-tissue window images of another patient with a right glomus jugulare tumor. The lesion shows avid enhancement. The axial image (b) also shows intimate contact with the right internal carotid artery (arrow). Fig. 3.17 Axial T2-weighted (a) and postgadolinium-enhanced T1-weighted axial (b) and coronal (c) images of a right glomus vagale tumor. The prominent flow voids are noted throughout the lesion. This tumor extends up into the jugular fossa. Note the presence of ipsilateral tongue fatty atrophy (asterisk; a) secondary to mass effect upon the hypoglossal nerve.
3.1 Basic Anatomy Overview
3.1.1 Cerebellopontine Angle Cistern
 Fig. 3.1). These walls may be deficient either as a normal variant or as part of a pathological process. Under normal circumstances, there is continuous exchange of CSF from one compartment to another through these openings which can be obstructed by certain conditions (e.g., subarachnoid hemorrhage, pus, and protein).1,2,3
Fig. 3.1). These walls may be deficient either as a normal variant or as part of a pathological process. Under normal circumstances, there is continuous exchange of CSF from one compartment to another through these openings which can be obstructed by certain conditions (e.g., subarachnoid hemorrhage, pus, and protein).1,2,3
Contents
 Fig. 3.2. To avoid confusion, we will discuss only the superior compartment of the CPA cistern.
Fig. 3.2. To avoid confusion, we will discuss only the superior compartment of the CPA cistern.
Boundaries
 Fig. 3.3).1,2,3
Fig. 3.3).1,2,3
3.1.2 Jugular Fossa
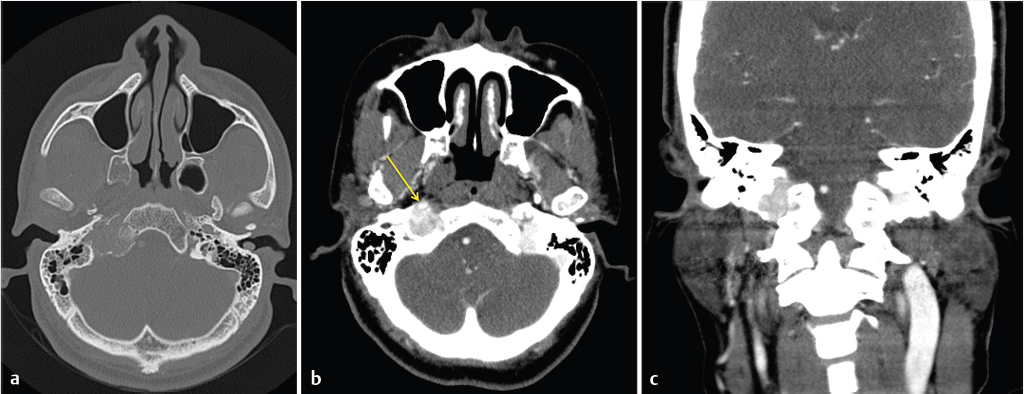
 Fig. 3.4).
Fig. 3.4).
 Fig. 3.5). The pars nervosa contains the inferior petrosal sinus and CN 9 with its tympanic branch (nerve of Jacobson). The latter can be occasionally seen on CT within the inferior tympanic canaliculus (which also contains the inferior tympanic artery) at the level of the caroticojugular spine (
Fig. 3.5). The pars nervosa contains the inferior petrosal sinus and CN 9 with its tympanic branch (nerve of Jacobson). The latter can be occasionally seen on CT within the inferior tympanic canaliculus (which also contains the inferior tympanic artery) at the level of the caroticojugular spine ( Fig. 3.6).4
Fig. 3.6).4
3.2 Lesions of the Cerebellopontine Angle
3.2.1 Vestibular Schwannoma
Imaging
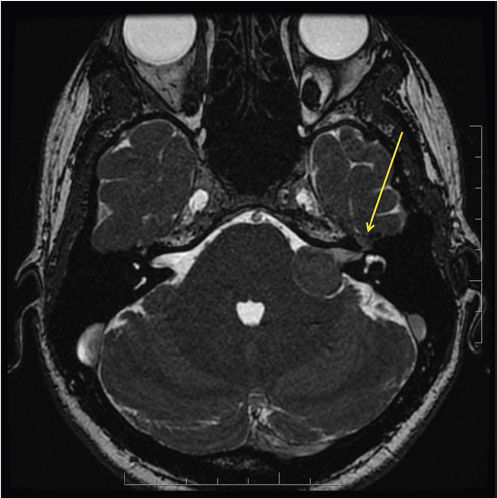
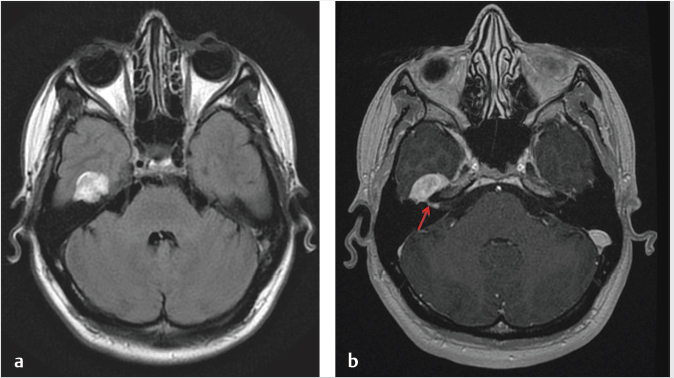
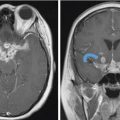
 Fig. 3.7). Valvassori observed an abnormal IAC on CT in 78% of pathologically proven VS. The difference between the abnormal and the normal IAC was found to be a canal height disparity (> 2 mm on the affected side), a shorter posterior wall of the canal (> 3 mm on the affected side), and a downward displacement of the crista falciformis.11
Fig. 3.7). Valvassori observed an abnormal IAC on CT in 78% of pathologically proven VS. The difference between the abnormal and the normal IAC was found to be a canal height disparity (> 2 mm on the affected side), a shorter posterior wall of the canal (> 3 mm on the affected side), and a downward displacement of the crista falciformis.11
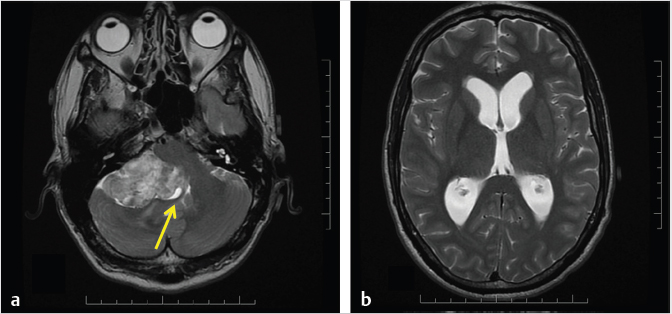
 Fig. 3.8). Giant VS may not have an intracanalicular component. The cisternal component of larger VS typically forms an acute angle with the petrous bone in 85% of cases (
Fig. 3.8). Giant VS may not have an intracanalicular component. The cisternal component of larger VS typically forms an acute angle with the petrous bone in 85% of cases ( Fig. 3.9).
Fig. 3.9).
 Fig. 3.9). Such findings are features that help identify patients who should be directed toward surgical decompression. When contrast MRI is used, a noncontrast (precontrast) fat-suppressed sequence should be routinely obtained to assess for areas of high signal intensity that reflect the presence of tumoral fat or hemorrhage. Such an inherent high T1 signal can also falsely mimic enhancement on the postcontrast sequences.
Fig. 3.9). Such findings are features that help identify patients who should be directed toward surgical decompression. When contrast MRI is used, a noncontrast (precontrast) fat-suppressed sequence should be routinely obtained to assess for areas of high signal intensity that reflect the presence of tumoral fat or hemorrhage. Such an inherent high T1 signal can also falsely mimic enhancement on the postcontrast sequences.
Imaging of VS post–radiation therapy
Prognosis and Treatment
3.2.2 Facial Nerve Schwannoma
Imaging
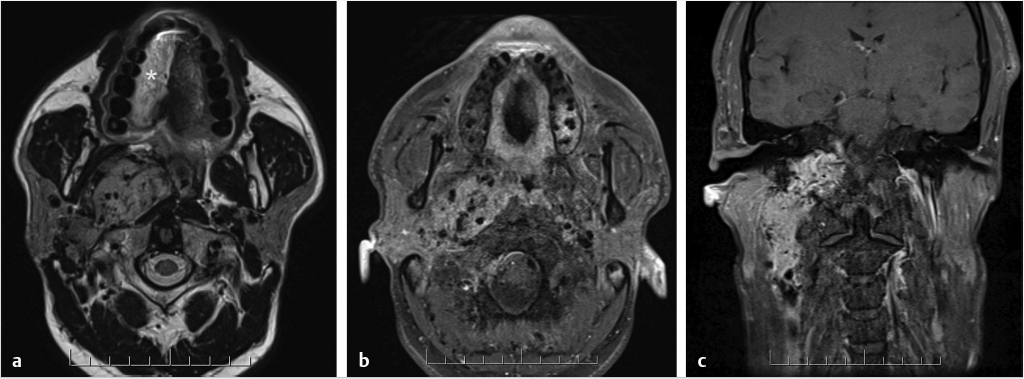
 Fig. 3.10). MRI features will parallel those on CT, such as, enlargement and enhancement of the facial nerve and geniculate ganglion (
Fig. 3.10). MRI features will parallel those on CT, such as, enlargement and enhancement of the facial nerve and geniculate ganglion ( Fig. 3.11).
Fig. 3.11).
 Fig. 3.12).
Fig. 3.12).
Prognosis and Treatment
3.2.3 Schwannomas along the Carotid Sheath
Imaging
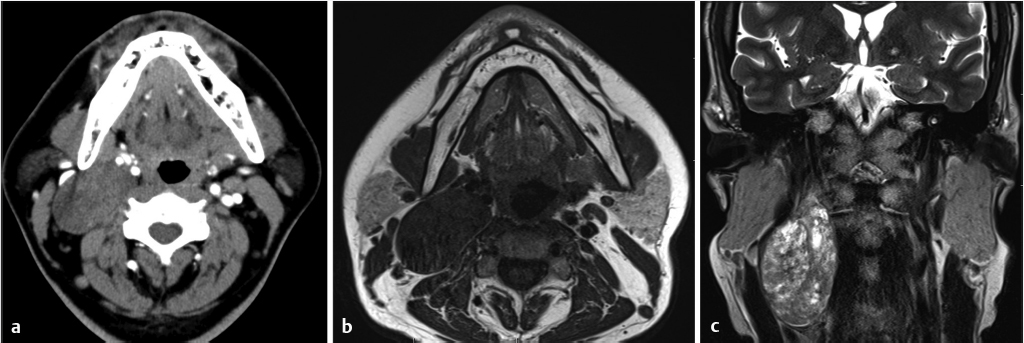
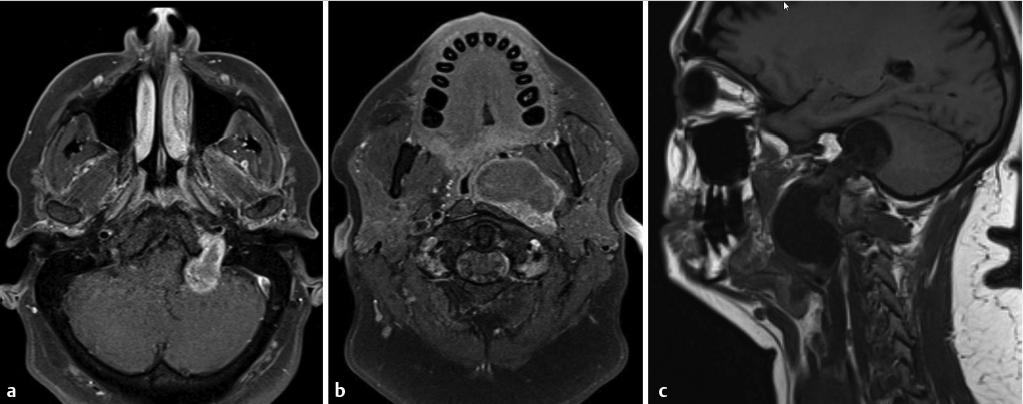
 Fig. 3.13,
Fig. 3.13,  Fig. 3.14).
Fig. 3.14).
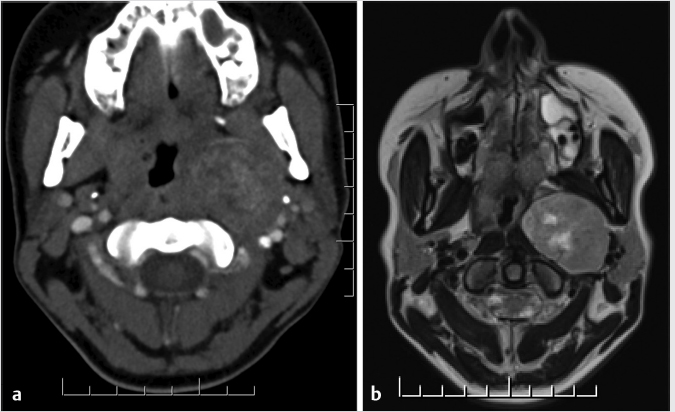
 Fig. 3.15). With the latter form of displacement, the appearance could mimic that of a salivary gland tumor arising either from the deep parotid lobe or originating primarily within the prestyloid PPS from extraparotid minor salivary cells.2,5,9
Fig. 3.15). With the latter form of displacement, the appearance could mimic that of a salivary gland tumor arising either from the deep parotid lobe or originating primarily within the prestyloid PPS from extraparotid minor salivary cells.2,5,9
Prognosis and Treatment
3.2.4 Paraganglioma (Glomus): Glomus Jugulare and Vagale
Imaging
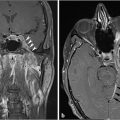
 Fig. 3.16). Actual vessels can occasionally be seen within the tumor. This is in contrast to schwannomas which are well-circumscribed lesions, less vascular, and frequently demonstrate cystic degeneration without bone destruction. A glomus vagale tumor will also appear as a vascular mass but will be positioned primarily in the poststyloid PPS, below the skull base. A glomus vagale tumor, however, can also grow to extend up into the JF (
Fig. 3.16). Actual vessels can occasionally be seen within the tumor. This is in contrast to schwannomas which are well-circumscribed lesions, less vascular, and frequently demonstrate cystic degeneration without bone destruction. A glomus vagale tumor will also appear as a vascular mass but will be positioned primarily in the poststyloid PPS, below the skull base. A glomus vagale tumor, however, can also grow to extend up into the JF ( Fig. 3.17).
Fig. 3.17).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree