- •
Enlarged heart, but with relatively preserved systolic function.
- •
No other obvious explanation as to why the heart is enlarged.
- •
Both right and left side of heart are enlarged.
- •
Dilated superior vena cava, out of proportion to the size of the inferior vena cava.
- •
Reversal of flow in the aortic arch in the absence of aortic insufficiency or other reason for aortic retrograde flow.
- •
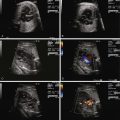
Visualization of lucent, vascular structure in the brain.
- •
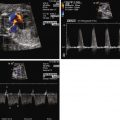
Increased middle cerebral artery systolic and diastolic flow.
- •
Mild or moderate tricuspid regurgitation.
Anatomy and Anatomical Associations
Cerebral arteriovenous malformations (AVMs) can occur as part of development of the cerebrovascular circulation and are generally quite rare. Although highly uncommon outside of prenatal life and infancy, the vein of Galen aneurysmal malformation is the most common type of cerebral AVM seen in the fetus.
The vein of Galen aneurysmal malformation (VGAM) is a choroidal type of AVM involving the vein of Galen forerunner and is distinct from an AVM with venous drainage into a dilated, but already formed, vein of Galen. The congenital malformation develops relatively early in gestation during weeks 6 to 11 of fetal development as a persistent embryonic prosencephalic vein of Markowski; thus, VGAM is actually a misnomer. The vein of Markowski actually drains into the vein of Galen. Nevertheless, the effect is one in which arterial blood bypasses capillary beds and is shunted directly to the venous system. VGAM can result in marked dilation of the straight and sagittal sinuses as well as the venous system draining to the heart.
VGAM typically result in high-output congestive heart failure in the fetus. The dilated venous structures may cause a mass effect leading to brain hypoplasia and altered cerebral development. Cerebral hemorrhage can also occur, and thrombosis in the fetus has been reported.
Interestingly, VGAM has been reported in association with two types of congenital heart disease—sinus venosus type atrial septal defect and coarctation of the aorta. The association is hypothesized to be related to early alterations in blood flow patterns as a consequence of the AVM. Increased superior vena caval return in utero may interfere with absorption of the right horn of the sinus venosus into the right atrium, leading to development of the defect. In addition, preferential flow toward the low-resistance carotid arteries feeding the AVM and away from the aortic isthmus may lead to decreased isthmul growth. Size discrepancy with marked dilation of the ascending and transverse aorta and relatively small isthmus can result in postnatal coarctation of the aorta.
Frequency, Genetics, and Development
VGAM is rare, but it is the most common type of cerebral AVM. It is a developmental abnormality that is completed and present by the first trimester. The effects relate to volume of blood flow; hence, dilation, cerebral compression, and cardiac volume overload may not be seen until the second or third trimester. There is no known associated genetic basis to this malformation.
Prenatal Physiology
As cerebral blood volume increases with gestation, an increasing amount of blood is shunted from the arterial to the venous side resulting in increased volume load to the cerebral venous system and, ultimately, increased volume load to the heart. The superior vena cava dilates as do the right ventricle and pulmonary artery. With volume loading of the right ventricle, the tricuspid annulus dilates, resulting in tricuspid regurgitation, which further adds volume load to the right heart. Blood ejected from the left side of the heart will take the pathway of least resistance up the ascending aorta and into the carotid arteries toward the low-resistance AVM connections. The head vessels will progressively enlarge. Blood flow in the isthmus of the aorta, or in the descending aorta, may flow retrograde in diastole as a “steal” phenomenon takes place, with blood being shunted into the fetal head, further increasing venous return.
Prenatal Management
Assessment of combined cardiac output can provide an overall measure of the potential degree of cardiovascular burden present in the VGAM. By measuring the diameter of the semilunar valves and sampling Doppler flow across the semilunar valves, one may calculate cardiac output for each ventricle. The normal combined cardiac output in the fetus is approximately 400 to 500 mL/kg/min of flow. In our experience, similar to what is seen in other volume-load lesions such as sacrococcygeal teratoma, the fetal cardiovascular system can tolerate volume loads as high as 700 to 800 mL/kg/min. However, values much higher than that typically lead to heart failure and hydrops.
The most common initial finding that prompts suspicion for VGAM is the identification of a large echolucent structure in the posterior aspect of the fetal head, midline behind the third ventricle. Application of color Doppler flow onto the structure may reveal very low velocity swirling of blood. At times, feeder vessels into the dilated venous sac can be seen.
No fetal therapeutic maneuvers are currently available for VGAM. Progressive findings of increased cardiac output and worsening volume overload with impending hydrops may prompt decisions toward delivery, balanced against gestational age.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree