Computed tomography (CT) and magnetic resonance imaging (MRI) have become indispensable for diagnosis, evaluation, and treatment of intracranial infectious disease, contributing to significant decline in morbidity and mortality of these patients. CT is useful as a rapid screening tool to detect complications of intracranial infections such as hydrocephalus, extraaxial collections, and mass effect. MRI is superior for assessment of intracranial infections and allows for a more sensitive evaluation of parenchymal lesions and leptomeningeal disease. CT angiography (CTA) is frequently used for detection of vascular complications such as vasculitis and mycotic aneurysms, with conventional cerebral angiography reserved for selected difficult cases and endovascular interventions. Implementation of advanced MRI techniques such as MR perfusion imaging, diffusion imaging, and proton MR spectroscopy (MRS) has improved differentiation of neoplastic processes from intracranial infections and allowed for more accurate characterization of these disorders. Functional MRI (fMRI) and diffusion tensor imaging (DTI) have allowed for improved preoperative planning when surgical intervention is necessary.
There are four common routes of spread of infectious disease to the intracranial compartment.
- 1.
Hematogenous spread is the most common route, with pathogens from a distant site in the body disseminating to the choroid plexus, meninges, and brain parenchyma. In cerebral parenchyma the pathogens usually lodge within small vessels, resulting in a subcortical distribution of such lesions. Anomalous hemodynamics, such as those seen in children with cyanotic heart disease, have a high incidence of intracranial infection secondary to a right-to-left shunt. Retrograde venous spread can also occur via anastomotic connections between superficial veins of the face, scalp, and cortical veins.
- 2.
Direct extension from an adjacent site of infection, such as mastoiditis, otitis media, sinusitis, or orbital infection is another possibility.
- 3.
Direct intracranial introduction of a pathogen may occur during trauma or as a complication of surgery or lumbar puncture.
- 4.
Retrograde spread to the central nervous system (CNS) via peripheral nerves can occur in herpes simplex and rabies virus infections.
Multiple coexisting conditions may increase the risk of intracranial infections (e.g., diabetes mellitus, alcoholism, malignancy, agammaglobulinemia, radiation therapy, chemotherapy, and steroid therapy). Patients with acquired immunodeficiency syndrome (AIDS) are particularly susceptible to intracranial infection; however, the disease spectrum and imaging manifestations in these patients are different.
A variety of infectious disorders involve the CNS. Pyogenic infection is discussed in detail in this chapter, with categorization according to the anatomic site of involvement. Other infectious processes are also discussed, including viral infections, granulomatous disease, spirochete infections, fungal diseases, parasitic diseases, and inflammatory disease.
Leptomeningitis
Three distinct layers of connective tissue known as the meninges surround the brain within the intracranial compartment. The dura mater layer is also referred to as the pachymeninges. The thinner inner arachnoid and pial meningeal layers are referred to as leptomeninges.
There are two principal patterns of meningeal enhancement on MRI: leptomeningeal and pachymeningeal enhancement. Because the dura mater lacks the blood-brain barrier, normal pachymeninges usually demonstrate only minimal discontinuous linear enhancement except for dural venous sinuses. The leptomeninges usually demonstrate no enhancement except for adjacent vascular structures.
Meningitis primarily involves the leptomeninges (pia mater and arachnoid membrane) and the adjacent subarachnoid space, resulting in a leptomeningeal pattern of enhancement. The diagnosis of meningitis is frequently made by clinical history and cerebrospinal fluid (CSF) analysis. Clinical presentation in adults may include the classic triad of fever, neck stiffness, and altered mental status, as well as headache and photophobia. CSF analysis in acute pyogenic meningitis classically demonstrates high protein, low glucose, and neutrophilic pleocytosis.
CT of the head should be obtained prior to lumbar puncture in patients with suspected increased intracranial pressure, focal neurologic deficits, or moderate to severe impairment of consciousness. Imaging may also be used to confirm meningitis and evaluate for complications such as abscess, ventriculitis, and subdural empyema. Additionally, cross-sectional imaging may elucidate the origin of intracranial infection, because CT and MRI are sensitive for sinusitis and mastoiditis.
The infectious agents responsible for acute pyogenic meningitis vary by age group and immune status. In neonates, causes include group B Streptococcus and Escherichia coli. In infants and young children, typical pathogens may include Haemophilus influenzae and Neisseria meningitides. In immunocompetent adults, Streptococcus pneumoniae and Listeria monocytogenes are the common causative agents. In immunocompromised adults, causative agents for acute meningitis include Klebsiella, Pseudomonas, E. coli, and fungal organisms.
Initial CT and MRI of the brain may be normal in uncomplicated cases of meningitis. Occasionally, in more advanced meningitis, noncontrast CT may demonstrate basal cistern obliteration. This may represent increased attenuation of exudate in the subarachnoid space and thickened, inflamed leptomeninges. Ventricular enlargement and diffuse cerebral swelling may also be seen in more advanced cases of meningitis. Postcontrast CT may show enhancement of leptomeninges in the basal cisterns and sylvian fissures.
MRI is more sensitive for detection of meningitis, especially when using fluid-attenuated inversion recovery (FLAIR) sequences. T2-FLAIR is sensitive to subtle changes in CSF and may demonstrate hyperintensity in the subarachnoid space (presumably due to exudate and elevated protein in this region) even when T1-weighted images may appear normal. However, T2-FLAIR sulcal hyperintensity may also be seen in other subarachnoid space processes such as leptomeningeal carcinomatosis and subarachnoid hemorrhage. Postcontrast T2-FLAIR has been reported to have high sensitivity and specificity for leptomeningeal enhancement.
T1-weighted images may show obliteration of the basal cisterns, and T2-weighted images may show cortical hyperintensity. Postcontrast T1-weighted images may show linear continuous sulcal or cisternal enhancement, frequently involving the basal cisterns or sylvian fissures and extending deep into the base of the sulci. Sulcal or cisternal enhancement on three contiguous slices has been reported to correlate with significant meningeal pathology ( Fig. 10-1 ).

On postcontrast MRI and CT imaging, differential diagnosis of abnormal subarachnoid enhancement includes meningitis, leptomeningeal carcinomatosis, and subacute subarachnoid hemorrhage, although the clinical presentation is usually very different in these entities. Contrast-enhanced MRI has been shown to be more sensitive than contrast-enhanced CT in detection of meningitis and its complications. Leptomeningeal enhancement on postcontrast MRI is seen in only 50% of meningitis cases.
Thin linear enhancement most evident along cerebral convexities is typically seen in acute pyogenic or viral meningitis. Nodular thick enhancement most evident in the basal cisterns is typically seen in chronic meningitides such as tuberculous meningitis, fungal meningitis, and sarcoidosis. Granulomatous disease may also result in pachymeningeal involvement.
Pachymeningeal thickening and enhancement may be seen in a variety of disorders including intracranial hypotension, meningiomas, metastatic disease, lymphoma, and granulomatous disease. Postoperative meningeal enhancement may be pachymeningeal or leptomeningeal in appearance. Occasionally, pachymeningeal enhancement can be seen in patients who had uncomplicated lumbar puncture.
Complications of meningitis include hydrocephalus, ventriculitis, subdural effusions, subdural empyema, epidural empyema, cranial nerve inflammation, cerebritis, abscess, and infarction due to arterial or venous involvement. Hydrocephalus is the most common complication and usually presents as transient communicating hydrocephalus due to subarachnoid space inflammation and secondary decreased CSF resorption in pacchionian granulations. Noncommunicating hydrocephalus may also occur, especially when meningitis is complicated by ventriculitis.
Magnetization transfer (MT) sequences have been reported to improve visualization of abnormal meninges by showing more prominent enhancement on postcontrast images. These sequences may also demonstrate basal cistern hyperintensity on noncontrast images in tuberculous meningitis. MT ratio (MTR) may help determine the cause of meningitis, with tuberculous meningitis reported to show lower MTR than fungal and pyogenic meningitis and higher MTR than viral meningitis.
Recent investigations of DTI have demonstrated high sensitivity in detecting bacterial meningitis, showing increased fractional anisotropy (FA) within enhancing and nonenhancing cortex. High FA is thought to be due to increased orientation of inflammatory cells in the pia-arachnoid meninges closely related to the cortical ribbon. FA of the cortex has also shown a strong correlation with neuroinflammatory markers in neonatal meningitis.
Subdural Effusion
Subdural effusion is a sterile fluid collection between the dura and the arachnoid. Reactive subdural effusions are common complications of acute bacterial meningitis, especially in infants. S. pneumoniae and H. influenzae meningitis are more frequently associated with this complication. These collections are similar to posttraumatic and postsurgical subdural effusions and may result from tears in the arachnoid membrane and subsequent leakage of CSF into the subdural space. Alternatively, subdural effusions may be caused by inflammation of subdural veins or dural irritation.
CT and MRI demonstrate extraaxial collections with attenuation and signal intensity similar to CSF, which are most frequently seen as large bilateral fluid collections over the frontal and temporal convexities. Subdural effusions may be isointense or hyperintense to CSF on FLAIR images, depending on protein concentration of the fluid. Lack of transiting venous structures within these collections on T2-weighted images allows for differentiation from prominent subarachnoid spaces. There is no diffusion restriction seen within these collections, and typically they show no significant enhancement. Occasionally, fibrin strands and membranes develop; they may enhance and appear similar to subdural empyema. Subdural effusions regress spontaneously and do not require intervention except when they are large and result in significant mass effect.
Subdural Empyema
Subdural empyema is a purulent collection between the dura and the arachnoid; it may arise as a complication of acute pyogenic meningitis, mastoiditis, otitis media, paranasal sinusitis, trauma, or postsurgical infection. The adjacent infection is thought to spread to the subdural space via retrograde thrombophlebitis of the calvarial emissary veins, bridging veins, or dural venous sinuses. More rarely, an infection of a subdural effusion may occur, resulting in a subdural empyema. These collections may cross the calvarial sutures but do not cross the midline.
Subdural empyemas represent up to 15% of all extraaxial collections and are seen in 13% to 20% of all cases of intracranial infection.
Subdural empyema may progress and result in multiple complications such as thrombophlebitis, dural venous sinus thrombosis, infarction, cerebritis, and abscess formation. For this reason it usually requires aggressive intervention, and early accurate diagnosis is critical.
CT imaging usually demonstrates a hypodense to isodense crescentic or lenticular extraaxial collection along the cerebral convexity or falx, which frequently appears similar to a subdural effusion. MRI is extremely useful when empyema is suspected, because it allows for reliable diagnosis of this entity and evaluation of associated complications.
Diffusion restriction is the most useful characteristic for diagnosis of empyema, which is seen as very high signal on diffusion-weighted images (DWI) and low values on apparent diffusion coefficient (ADC) maps. In contradistinction, subdural effusions show signal intensity similar to CSF on DWI. Empyemas are also typically slightly hyperintense to CSF on T1-weighted images and usually demonstrate rim enhancement ( Fig. 10-2 ). The presence of adjacent cerebritis, abscess, or infarction with associated parenchymal enhancement or diffusion restriction also supports the diagnosis of empyema. Hyperintense signal on T2-FLAIR and T2-weighted images compared to CSF is not specific, because it may be seen in subdural effusions and empyemas.

Epidural Empyema
Epidural empyema, or epidural abscess, is a purulent collection between the dura and the calvarium and usually occurs as a complication of otitis media, mastoiditis, sinusitis, or osteomyelitis of the skull. Ten percent of epidural empyemas may be associated with subdural empyema.
Epidural empyema is separated from the subarachnoid space and brain parenchyma by the dura, which may act as a barrier to infection. As a result this collection usually has a more insidious and benign clinical course. Owing to its extradural location, this collection usually has biconvex morphology and may cross and displace the dural venous sinuses but does not cross the calvarial sutures. Typically an avidly enhancing T2 hypointense rim is seen. Adjacent parenchymal involvement is rare ( Fig. 10-3 ). Imaging characteristics are otherwise similar to subdural empyema.

Ependymitis
Ependymitis or ventriculitis is inflammation of the ependyma along the inner surface of the cerebral ventricles. Intraventricular infection may result from intraventricular rupture of a cerebral abscess, hematogenous spread to subependyma or choroid plexus, retrograde spread from extraventricular CSF spaces, trauma, or surgery. Staphylococcus and Enterobacter are the most common causative agents. Imaging plays an important role in diagnosis; signs and symptoms may be subtle.
Noncontrast CT frequently shows no significant abnormality, although occasionally dependent debris or slightly hyperdense ependyma may suggest the diagnosis. MRI findings include enhancing thickened ependyma, T2 hyperintense ventricular walls, dependent intraventricular debris, and ventricular enlargement. Dependent debris is a characteristic finding in ventriculitis and usually demonstrates diffusion restriction and hyperintensity on T2-FLAIR images ( Fig. 10-4 ). Intraventricular adhesions and septations may cause noncommunicating hydrocephalus or result in a trapped ventricle or segmental dilatation.

Cerebritis and Abscess
Cerebritis is a poorly defined regional acute inflammation of cerebral parenchyma with increased local vascular permeability, which is usually caused by pyogenic bacteria. Untreated cerebritis evolves into a cerebral abscess, which is characterized as a focal parenchymal collection of pus with a surrounding vascular collagenous capsule.
Britt and Enzmann divided the process of abscess formation into four stages: early cerebritis, late cerebritis, early capsule formation, and late capsule formation.
- 1.
Early or acute cerebritis (first 3-5 days). Bacteria invade the parenchyma and trigger an immune response, with infiltration by inflammatory cells, increased vascular permeability of the blood-brain barrier, perivascular exudates, petechial hemorrhages, microthrombosis, early necrosis, and associated edema.
Imaging is rarely performed at this stage, because the patient may still be asymptomatic. CT shows a poorly defined area of parenchymal hypodensity, with mass effect and variable enhancement, including no enhancement, mild nodular, or ring enhancement. MRI is more sensitive for detection of cerebritis and abscess. A poorly defined parenchymal area of edema with T2 hyperintensity and T1 iso- to hypointensity is seen with variable ill-defined enhancement ( Fig. 10-5 ).

FIG 10-5
Early cerebritis. A, T2-weighted image demonstrates two ill-defined areas of high signal intensity involving the cortex and subcortical white matter of the left frontal lobe. B, Gadolinium-enhanced MRI study shows mild ill-defined peripheral enhancement. Subsequent imaging (not shown) demonstrated formation of two mature abscesses in these areas.
- 2.
Late cerebritis (second week). There is progression of necrosis with formation of a large necrotic zone. Peripheral fibroblast deposition occurs, with formation of reticulin matrix.
MRI and CT demonstrate thick, irregular, ring enhancement around a central necrotic zone with a surrounding area of edema. Central necrosis shows decreased attenuation on CT, with T1 hypointensity, T2 hyperintensity, and diffusion restriction on MRI. A rim may be seen around the necrotic zone on MRI, which is T1 iso- to hyperintense and T2 iso- to hypointense ( Fig. 10-6 ).

FIG 10-6
Late cerebritis. A, Gadolinium-enhanced MRI study shows thick, smooth, ringlike enhancement with surrounding edema. B, Gadolinium-enhanced MRI study (coronal view) shows a second small adjacent ringlike enhancement.
- 3.
Early abscess — early capsule formation (end of second week). Granulation tissue forms at the periphery of the abscess. A thin capsule is formed by fibroblasts, with reticulin matrix and scant collagen. Liquefaction occurs in the central necrotic zone, with reduction in surrounding edema.
CT frequently demonstrates iso- to slightly hyperdense capsule interposed between the hypodense central area and surrounding hypodense edema ( Fig. 10-7 ). MRI demonstrates a well-demarcated T2 hypointense capsule surrounding a central T2 hyperintense area, demonstrating diffusion restriction. Typically there is well-defined avid enhancement of the capsule, which may be thinner on the side closer to the ventricle ( Fig. 10-8 ). Daughter abscesses may appear at this stage.

FIG 10-7
Mature abscess. Noncontrast CT demonstrates a central hypodense cavity with an iso- to slightly hyperdense rim and surrounding edema.

FIG 10-8
Mature abscess. A, T2-weighted image shows a mature abscess with a hypointense rim, central cavity, and adjacent surrounding edema. B, Diffusion-weighted image demonstrates internal high signal intensity. Corresponding ADC images (not shown) demonstrated low values representing diffusion restriction. C, T1-weighted image demonstrates hypointense central cavity with iso- to hyperintense rim. D, T1-weighted postcontrast image demonstrates smooth ringlike enhancement corresponding to the hypointense rim seen on T2-weighted image.
- 4.
Mature abscess — late capsule formation (after second week). A thick collagen capsule is formed, with surrounding gliosis. The wall of the mature abscess consists of three layers: (1) an inner inflammatory layer of granulation tissue containing macrophages, (2) a middle collagenous layer, and (3) an outer gliotic layer.
The capsule may become thinner and better defined as surrounding edema decreases. Over time and especially with treatment, the capsule may become less T2 hypointense, enhancement may decrease, and the abscess may gradually decrease in size.
Cerebral abscesses are caused by multiple mechanisms, including direct spread from a local infection, hematogenous dissemination from a remote infection, trauma, or postsurgical infection. Up to 30% of cerebral abscesses are reported to be cryptogenic. Sources of direct spread include sinusitis, otitis media, mastoiditis ( Fig. 10-9 ), and odontogenic infections, with pathogens invading the brain parenchyma via adjacent osteomyelitis or via emissary veins.

Sources of hematogenous seeding include endocarditis, pulmonary abscesses, and abdominal infections. Congenital cyanotic heart disease and pulmonary arteriovenous malformation patients are at high risk for hematogenous dissemination of infections to the brain.
Presenting symptoms depend on size and location of the abscess and may include headache, fever, nausea and vomiting, neurologic deficits, and seizure. Absence of leukocytosis and normal CSF analysis do not exclude cerebral abscess, and CSF cultures are often sterile. Infectious agents include microaerophilic streptococci, anaerobic bacteria, Staphylococcus aureus, facultative gram-negative bacteria, and polymicrobial infections. Nocardial abscesses have been associated with high mortality.
There are three characteristic findings of a cerebral abscess on MRI: (1) central liquefied cavity with diffusion restriction; (2) smooth, thin, continuous capsular enhancement; (3) T2 hypointense signal in the capsule. Frequently there is thinning along the medial wall of the capsule. The capsule is usually T1 iso- to hyperintense compared to gray matter (see Fig. 10-8 ). Usually only a moderate amount of edema is seen surrounding a mature abscess. Gas-forming organisms may result in gas accumulation within the central liquefied zone.
Typical T2 hypointensity of the capsule is thought to be due to the presence of free radicals, which are generated by macrophages within the wall of the abscess (see Fig. 10-8A ). Satellite lesions associated with an abscess also support the diagnosis ( Fig. 10-10 ). Clinical improvement is correlated to decrease in enhancement and surrounding edema on CT and MRI.

Complications of cerebral abscess include hydrocephalus, meningitis, subdural empyema, and thrombophlebitis. Ependymitis resulting from rupture of an abscess into the ventricular system is a severe complication with high mortality. The medial wall of the abscess is often thinner owing to less extensive blood supply in periventricular white matter, which predisposes an abscess to rupture medially ( Fig. 10-11 ).

The classic ring enhancing appearance of cerebral abscess is not specific. CT and MRI imaging differential diagnosis includes high-grade primary CNS neoplasm, metastasis, nonpyogenic infection, radiation necrosis, demyelinating lesion, thrombosed aneurysm, subacute infarct, and resolving hematoma. Hematoma demonstrates typical MRI signal patterns of blood products, and infarcts demonstrate gyriform enhancement or cortical laminar necrosis, leading to the correct diagnosis. Neoplasms and granulomatous diseases typically have thicker and more nodular enhancement. Generally the presence of characteristic imaging features of cerebral abscess offers high specificity and sensitivity for this diagnosis. The presence of central diffusion restriction (hyperintensity on DWI and low values on ADC map) is particularly important in differentiation of abscess from other pathology and has been reported to have sensitivity of 95.2% and specificity of 95.7% ( Fig. 10-12 ; see also Fig. 10-8 ). In contradistinction, necrotic tumors usually demonstrate central diffusion facilitation.

Diffusion restriction within a liquefied center of an abscess is now thought to be primarily due to high density of viable inflammatory cells in pus, with high viscosity, macromolecules, and necrotic debris as possible secondary contributors. Recent investigations suggest that diffusion imaging may be helpful to follow up evaluation of treatment response, with high ADC reported in treated abscesses and low ADC after therapy reported in treatment failure.
The features of abscess tend to differ in immunocompromised patients receiving steroids. In these patients, edema may be minimal and the abscess capsule may be thick and irregular. Hypointensity of the capsule seen on T2-weighted images may not be obvious. Extraparenchymal spread into the ventricles and meningitis are more common.
Thallium-201 has been investigated for differentiation of abscess from necrotic tumor. However, a report of a false-positive result with uptake in a brain abscess suggests that this technique may not be reliable. However, this technique has been shown to be useful in differentiation of CNS lymphoma from Toxoplasmosis gondii infection in HIV patients.
Advanced MRI imaging techniques such as 1 H MRS, DTI, and MR perfusion have been recently employed to assist in differentiation of cerebral abscess from other lesions such as neoplasms with central necrosis.
The most characteristic MRS findings within the liquefied nonenhancing center of a mature cerebral abscess include presence of cytosolic amino acids (valine, leucine, and isoleucine) at 0.9 ppm, lactate at 1.3 ppm, and absence of normal metabolites such as N -acetylaspartate (NAA), choline, and creatine. Acetate at 1.9 ppm and succinate at 2.4 ppm may also be present. Lipid and lactate are not specific and may be seen in tumors and abscesses. Detection of amino acids is the most useful finding for diagnosis of abscess; these metabolites are not detected in vivo in necrotic or cystic neoplasms. Amino acids are generated during protein breakdown in pus owing to release of proteolytic enzymes by neutrophils. Amino acids, acetate, and succinate are generated during glycolysis and may be detected in abscesses caused by anaerobes or facultative anaerobes. Amino acids, acetate, and succinate have been reported to become undetectable after treatment.
Investigation of MR perfusion by Chiang et al. demonstrated lower mean relative cerebral blood volume (rCBV) in the enhancing wall of an abscess when compared to the enhancing rim of a necrotic tumor; this is due to the relatively poor vascularity of the abscess wall.
Recent studies using DTI in brain abscess showed increased fractional anisotropy (FA) within the abscess’s liquefied center. High FA is thought to be due to organized orientation of inflammatory cells, caused by neuroinflammatory molecules in pus, which may assist in differentiation from a necrotic tumor. Additionally DTI may be useful for evaluation of treatment response, because FA has been reported to decline during treatment, while mean diffusivity (MD) was relatively unchanged.
Encephalitis
Encephalitis is a diffuse inflammation of the brain with clinical evidence of brain dysfunction, typically caused by a viral infection. Infectious encephalitis may present with fever, headache, and altered level of consciousness. Viral causes include herpes simplex virus type 1 (HSV-1), herpes zoster, arbovirus, and enteroviruses in adults; and herpes simplex virus type 2 (HSV-2), cytomegalovirus (CMV), rubella, and human immunodeficiency virus (HIV) in neonates. In immunocompromised patients, common viral causes include HIV, CMV, John Cunningham virus (JC virus), and human herpesvirus 6 (HHV-6). Nonviral infectious encephalitis may also be seen in immunocompromised patients, with causative agents such as Toxoplasma, Aspergillus, and Listeria monocytogenes. Noninfectious encephalitides of autoimmune etiology include numerous entities such as acute disseminated encephalomyelitis (ADEM), Rasmussen encephalitis, and anti-NMDAR-mediated encephalitis.
Herpes Simplex Encephalitis
Herpes Simplex Type 1 (HSV-1) Encephalitis.
HSV-1 is estimated to cause 10% of the encephalitis cases in the United States and is the most common cause of sporadic encephalitis worldwide. HSV-1 encephalitis occurs in children, adults, and immunocompromised patients. Infection occurs early in life, with virus remaining in latent form in the vagus nerve, sacral nerves, and trigeminal ganglia. Reactivation later in life may result in rapidly progressive encephalitis with headache, fever, seizures, and altered mental status. Untreated HSV-1 encephalitis may result in death within 2 weeks, with a mortality rate of approximately 70%. For this reason early diagnosis is critical, with immediate treatment with acyclovir in clinically suspected cases.
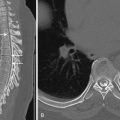
Characteristic findings on MRI include bilateral asymmetric T2-FLAIR hyperintensities in the cortex and subcortical white matter, in addition to sulcal effacement involving the hippocampi and mesial temporal lobes. The inferior frontal lobes, insula, and cingulate gyri may also be involved. The basal ganglia are spared, helping in differentiation from other encephalitides that frequently involve the basal ganglia. Patchy diffusion restriction may be seen early and may precede T2-FLAIR hyperintensities. Petechial hemorrhages may be demonstrated in areas of involvement. Later in the course of disease, patchy enhancement may also be seen ( Fig. 10-13 ; see also Fig. 10-12 ).

CT scan may show only minimal changes, with loss of gray-white matter junction, sulcal effacement, and hypodensity in the areas of involvement. MRS frequently demonstrates decreased NAA, increased choline, and increased lactate. Definitive diagnosis is established by CSF polymerase chain reaction (PCR) or brain biopsy. Chronic sequelae of HSV-1 encephalitis may include volume loss, encephalomalacia, and calcifications within involved regions of the brain.
Herpes Simplex Type 2 (HSV-2) Encephalitis.
HSV-2 causes 80% of neonatal HSV infections and is transmitted from mother to neonate, most frequently during delivery as the baby passes through the vaginal canal. More rarely, infection may occur in utero or in the postpartum period. Infection may result in skin, eye, or mouth involvement, encephalitis, or disseminated disease.
Typical imaging presentation includes T2-FLAIR hyperintensity involving basal ganglia as well as the cortex and subcortical white matter in the temporal and frontal lobes. T2 hyperintensity may be demonstrated in white matter diffusely. Contrast enhancement is often seen in these areas. In early encephalitis only diffusion restriction may be evident. Hemorrhages may be seen later in disease. Chronic sequelae include atrophy with cystic encephalomalacia and ventricular enlargement.
When acquired in utero, the patient may present with cataracts, microcephaly, cerebral calcifications, and ventricular enlargement as a part of TORCH syndrome. Diagnosis is made by CSF PCR or detection of virus in mucocutaneous lesions. Acyclovir therapy is recommended in suspected cases.
Human Herpesvirus 6 (HHV-6) Encephalitis
HHV-6 infection also occurs early in life, with virus remaining in latent form in immunocompetent adults. HHV-6 reactivation and subsequent encephalitis, pneumonitis, and bone marrow suppression may occur in transplant recipients. Imaging findings of HHV-6 encephalitis are similar to HSV-1 encephalitis, except that in HHV-6 encephalitis, contrast enhancement is not demonstrated.
Varicella Zoster Virus (VZV)
VZV is a herpesvirus usually causing infection in childhood and presenting as chickenpox (varicella) and subsequently remaining in latent phase in ganglionic neurons, especially in dorsal root ganglia. In children, VZV encephalitis may occur as a complication of chickenpox or later reactivation. VZV encephalitis resulting from vaccine has been reported. In adults, VZV reactivation presents as shingles (zoster) as the virus travels along a sensory nerve and results in a painful rash. Ramsay Hunt syndrome occurs with reactivation of VZV in the geniculate ganglion of cranial nerve VII, resulting in facial weakness and a rash known as zoster oticus. MRI may demonstrate facial nerve enhancement.
VZV reactivation may occur in older immunocompetent patients or in immunosuppressed patients and may also result in vasculopathy, retinal necrosis, cerebellitis, leukoencephalopathy, and myelopathy. CNS involvement may occur in patients without skin lesions. Acyclovir is used for treatment of VZV.
Vasculopathy is thought to be the dominant component of cerebral involvement by VZV, although meningoencephalitis may also be seen. Large- and small-vessel arterial involvement may be present. Clinical manifestations include infarcts and transient ischemic attacks, headaches, cranial neuropathies, and neurologic deficits.
Vasculitis may be demonstrated on MR angiography (MRA), CT angiography (CTA), or digital subtraction angiography (DSA). Characteristic findings include multiple vessels with segmental narrowing ( Fig. 10-14 ). Brain imaging features of VZV involvement are variable and may show T2-FLAIR hyperintensities at the gray-white matter junction and less typically localizing to white matter or gray matter. Enhancing lesions and infarcts may also be seen. Rarer sequelae include aneurysm formation, dissection, and hemorrhage (parenchymal or subarachnoid).

In children, VZV encephalitis may occur 10 days after appearance of the rash. MRI shows T2 hyperintense lesions at the gray-white matter junction, cortex, basal ganglia, and cerebellum. Vasculitis and associated infarction may occur 1 to 4 months later. VZV may also cause acute cerebellitis and present with ataxia after infection or vaccination. Numerous other viruses such as Epstein-Barr virus (EBV), HSV-1, HHV-6, enteroviruses, measles, and mumps may cause cerebritis with similar clinical and radiologic presentations. Usually symptoms resolve over weeks to months. MRI demonstrates diffuse bilateral cerebellar hemisphere T2-FLAIR hyperintensity and diffusion restriction.
Arbovirus Encephalitis
Arboviruses are viruses that are transmitted by arthropod vectors such as ticks and mosquitoes to humans. Arboviral encephalitides include West Nile virus (WNV) encephalitis, eastern and western equine encephalitis, St. Louis encephalitis, and Japanese encephalitis (JE), with varying epidemiology, morbidity, and mortality in these entities. Arboviral encephalitides have no specific treatment, and imaging may help differentiate these diseases from treatable encephalitides caused by HSV and VZV.
WNV is transmitted by a mosquito vector, with 1 out of 150 infections resulting in meningitis or encephalitis. MRI findings are nonspecific and include T2 hyperintensities in the basal ganglia, thalami, and brainstem, with frequent associated diffusion restriction. Mesial temporal lobe and long segment of spinal cord involvement may also occur.
JE virus is a leading cause of viral encephalitis in Asia but is less common in the United States. Imaging findings are similar to WNV encephalitis, with bilateral T2 hyperintensities in thalami and brainstem. Bilateral thalamic hemorrhages are characteristic for this disease. Although the mesial temporal lobe may be involved, JE spares the anterior temporal lobe and insula.
Acute Disseminated Encephalomyelitis (ADEM)
ADEM is an autoimmune demyelinating disease that usually occurs 2 to 31 days following a viral infection or vaccination. This is usually a monophasic disease, but a less common multiphasic form may be seen with relapses occurring shortly after presentation, known as multiphasic disseminated encephalomyelitis (MDEM). Autoimmune response to myelin is thought to be responsible for ADEM.
MRI is superior to CT for detection of ADEM lesions. Typically multiple bilateral, asymmetric, poorly defined, T2-FLAIR hyperintense, nonenhancing lesions are seen in the subcortical white matter. The corpus callosum and periventricular white matter are usually spared in contradistinction to multiple sclerosis (MS). Gray matter involvement is frequently seen in the thalami, basal ganglia, and cortex. Brainstem and spinal cord lesions may also be present. Occasionally contrast enhancement may be seen associated with the lesions. All lesions usually have similar imaging features due to the typical monophasic course of ADEM and characteristically show improvement after treatment with steroids ( Fig. 10-15 ). Imaging differential diagnosis includes MS and vasculitis, as well as infectious and metabolic disorders

Other Encephalitides
Creutzfeldt-Jakob Disease (CJD).
CJD is a transmissible spongiform encephalopathy presenting as progressive dementia. CJD is caused by prions, which are abnormal protein particles that induce abnormal folding of normal proteins in the brain, resulting in neuronal loss and spongiform changes typical of CJD. Three forms of the disease are recognized: sporadic, familial, and acquired. Acquired forms include kuru, iatrogenic CJD, and new variant CJD. The new variant of CJD is due to bovine spongiform encephalopathy (so-called mad cow disease). Iatrogenic CJD may result from use of contaminated human growth hormone, corneal transplantation, and cadaveric dural matter transplantation. Sporadic CJD with no known source of infection occurs in adults in their late 50s and accounts for most cases (≈90%). Rapidly progressive dementia leading to death within several weeks to months and myoclonus are usually seen. Synchronous discharges on EEG are seen in the sporadic form.
In sporadic CJD, MRI typically demonstrates diffusion restriction of the basal ganglia, thalami, cortex of the insula, cingulate gyri, superior frontal gyri, and other areas near the midline ( Fig. 10-16 ). Associated T2 hyperintensity may be also seen in these regions in some cases. MRS is nonspecific, demonstrating decreased NAA. The new variant or bovine spongiform encephalopathy may present with bilateral pulvinar T2 hyperintensities known as pulvinar sign or with a combination of dorsomedial thalamic nucleus and pulvinar hyperintensity known as hockey stick sign .

Subacute Sclerosing Panencephalitis (SSPE).
SSPE affects older children and young adults 6 to 8 years after a primary measles infection. This subacute encephalitis progresses through four clinical stages and usually results in death within 2 to 4 years. Early stages present with personality and behavioral changes, incoordination, tremor, and myoclonus and may show no imaging abnormalities. Late-stage disease results in coma, and MRI may demonstrate hyperintensities on FLAIR and T2-weighted images in periventricular white matter, splenium of the corpus callosum, thalami, basal ganglia, brainstem, and cerebellum. Cerebral and cerebellar atrophy may be seen on CT and MRI.
Reye’s Syndrome.
Reye’s syndrome is a noninflammatory encephalopathy and liver dysfunction that typically presents after an upper respiratory tract infection and is associated with use of salicylates. This entity is now rarely seen, in part because of a decline in pediatric aspirin use. MRI may reveal cerebral edema and T2 hyperintensities with diffusion restriction in the midbrain, thalamus, cortical, and subcortical regions. Pontine T2 hyperintensity may also be demonstrated. CT may show diffuse cerebral edema.
Encephalitis in Immunocompromised Patients
HIV Encephalitis.
HIV causes a subacute encephalitis through direct involvement of the brain and may result in a spectrum of clinical presentations, including neurocognitive abnormalities and AIDS dementia complex (ADC). ADC, a progressive dementia with behavioral and motor abnormalities, has become less common with the advent of highly active antiretroviral therapy (HAART). However, mild neurocognitive disorders remain common. Typical MRI manifestations include cerebral atrophy and symmetric patchy or confluent periventricular white matter lesions, sparing the subcortical U-fibers, which are T2-FLAIR hyperintense and T1 isointense. CT is less sensitive, and areas of abnormal white matter may be seen as hypodensities on CT in advanced disease. Severity of atrophy has been shown to correlate with severity of neuropsychiatric abnormalities.
MRS shows decreased NAA, elevated choline, and elevated myoinositol. Investigations of this entity using DTI have reported increased MD and decreased FA in normal-appearing white matter of HIV-infected individuals with detectable viral loads. However, a more recent study found DTI not to be useful for detection of early HIV infection in the brain.
HIV may also cause vacuolar myelopathy of the cord, presenting with bilateral T2 hyperintense dorsal columns extending over multiple segments. These findings are similar to subacute combined degeneration in B 12 deficiency.
Progressive Multifocal Leukoencephalopathy (PML).
PML is caused by reactivation of a latent JC virus, a human polyomavirus that infects oligodendroglial cells of the brain. JC virus infects a majority of the population early in life, persisting in latent form and occasionally reactivating with immunosuppression. PML occurs in AIDS and less frequently in hematologic malignancies or with immunosuppressive medications, including therapy for transplant patients and MS patients on natalizumab. Patients present with altered mental status, progressive neurologic deficits, and lethargy. Without treatment, death may result within 6 to 12 months.
Imaging findings include multiple bilateral, asymmetric, and often confluent subcortical and periventricular white matter lesions that are T2-FLAIR hyperintense, T1 hypointense, and hypodense on CT. Lesions may show central diffusion facilitation with peripheral diffusion restriction and usually show no significant enhancement ( Fig. 10-17 ). Occasionally faint peripheral enhancement is seen, which is associated with favorable outcomes. The lesions may involve any part of the brain, including posterior fossa structures, but they more frequently involve the supratentorial brain, basal ganglia, and thalami. PML lesions usually involve subcortical U-fibers, which are spared in HIV encephalopathy. Additionally, PML lesions are frequently T1 hypointense, whereas HIV lesions are isointense to white matter. MTR is markedly reduced in PML lesions compared to HIV lesions; presumably this is due to myelin macromolecule destruction in PML. MRS shows decreased NAA, increased choline, and possibly the presence of lactate and lipid. Diagnosis may be confirmed with CSF PCR.

Immune reconstitution inflammatory syndrome (IRIS) represents worsening of neurologic deficits following initiation of therapy and is due to recovery of the immune system and enhanced inflammatory response to infection. PML-IRIS may occur in 23% of HIV-associated PML and may also be seen in MS patients on monoclonal antibody therapy. Steroid therapy may be useful in this setting.
MRI reveals progression of findings a few weeks following initiation of therapy, with contrast enhancement in lesions, increased lesion size, increased edema, and mass effect. These findings later improve and resolve.
Cytomegalovirus (CMV) Encephalitis.
CMV is a ubiquitous DNA virus in the Herpesviridae family and is present in latent form in a majority of the general population. In immunocompetent patients it may cause a self-limited febrile illness. In immunocompromised patients CMV may cause disseminated disease, including respiratory, gastrointestinal (GI), and CNS infections. CMV may cause ventriculitis, meningoencephalitis, retinitis, radiculomyelopathy, and peripheral neuropathy. CMV encephalitis may be classified in two forms that may be concurrent: microglial nodular encephalitis and ventriculoencephalitis.
Microglial nodular encephalitis is similar to HIV encephalitis, causing progressive dementia. Unlike HIV encephalitis, progression is more rapid and both cranial nerve palsies and disorientation may occur. Imaging may be unremarkable or may show periventricular white matter T2-FLAIR hyperintensities and atrophy similar to HIV encephalopathy. Ventriculoencephalitis is a subacute necrotizing infection of the ependyma and subependymal tissue presenting as ataxia, cranial nerve palsies, progressive confusion, and lethargy, resulting in death within weeks. MRI demonstrates ependymal and subependymal T2-FLAIR hyperintensity and enhancement. However, imaging findings may be subtle or absent in some cases. Rarely meningeal enhancement or a ring enhancing mass lesion may be seen. CT is less sensitive, showing hypodensity in the periventricular white matter and atrophy. Quantitative CSF viral PCR is used to establish the diagnosis.
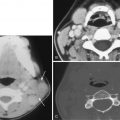
CMV is the most common cause of congenital infection in the United States and may be acquired by transplacental transmission, during birth, or through breast milk ingestion. CNS involvement may result in microcephaly, chorioretinitis, hearing impairment, cerebral palsy, epilepsy, and mental retardation. Most severe damage occurs in first and second trimester infections, resulting in lissencephaly, polymicrogyria, small cerebellum, and ventriculomegaly. Later infections result in white matter injury, with T2-FLAIR hyperintensity, periventricular calcifications, ventriculomegaly, and periventricular cysts. Subependymal distribution of calcifications differs from the randomly scattered distribution of calcifications seen in congenital toxoplasmosis ( Fig. 10-18 ).

Septic Embolus and Mycotic Aneurysm
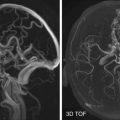
Infective endocarditis is most frequently caused by Staphylococcus aureus, and its major complication is septic emboli and stroke. Other causes of septic emboli to the brain include pulmonary infection, pulmonary arteriovenous malformation with sepsis, intravenous drug abuse, and cyanotic heart disease. The majority of septic emboli cases are seen in patients with a history of drug abuse. Intracranial septic emboli may lead to formation of mycotic aneurysms, abscess formation, or obliterative vasculitis. Mycotic aneurysms probably result from infarction of the arterial wall secondary to infected emboli. Mycotic aneurysms may be solitary or multiple, and they usually involve middle cerebral artery branches. The relative frequency of middle cerebral artery branch involvement is probably explained by the likelihood of emboli being carried into this territory.
Rupture of a mycotic aneurysm may produce an intracerebral or subdural hematoma ( Fig. 10-19 ). An unruptured mycotic aneurysm may occasionally be detected by contrast-enhanced CT or MRI studies. Associated infarction or small areas of cerebritis or abscess formation may be seen on imaging. Fungal infectious aneurysms frequently result from craniofacial infections. Typically they present as large proximal vessel fusiform aneurysms and are associated with a worse prognosis (see Fig. 10-29 ).