Cervical and Uterine Cancer
Perry W. Grigsby
The initial diagnosis and staging of most gynecologic malignancies are commonly achieved by history and physical examination and by use of selected imaging studies. Accurate staging of gynecologic cancers is important both for selecting appropriate therapy and for prognosis. Most gynecologic cancers initially spread regionally and then through lymphatic channels before hematogenous dissemination to distant organs. With locally advanced disease, the status of pelvic and para-aortic lymph nodes is an important determinant of prognosis and guides treatment planning decisions. Computed tomography (CT) has been the most widely used imaging method for assessment of nodal involvement and detection of distant metastatic disease. Despite its high resolution and excellent depiction of anatomy, CT is limited by its inability to detect small- volume metastatic involvement in normal-sized lymph nodes and to determine whether enlarged nodes represent metastasis or reactive hyperplasia (which is particularly problematic in patients with large necrotic tumors with significant associated inflammation). Recognition of small peritoneal tumor deposits is also difficult on CT.
Positron emission tomography (PET) with the glucose analogue 2-fluorine-18-fluoro-2-deoxy-D-glucose (FDG) has become an established imaging tool for many cancers. The functional information about regional glucose metabolism provided by FDG PET provides for greater sensitivity and specificity in most cancer imaging applications by comparison with CT and other anatomic imaging methods. The role of PET in gynecological cancers is evolving, but the current literature suggests that PET is superior to conventional imaging modalities for evaluating patients with cervical cancer. The role of PET in other gynecological cancers is less well defined.
The development and rapid dissemination of integrated PET/CT scanners that allow functional and anatomical information to be obtained in a single examination represents an important advance in PET imaging technology, resulting in a synergistic improvement in the accuracy of interpretation of both PET and CT images (1). Accordingly, the performance of PET based on published studies performed with conventional PET scanners is now being re-evaluated in light of emerging PET/CT results (2).
Patient Preparation and Imaging
Patient preparation for PET imaging of gynecological tumors is similar to that for PET oncologic imaging. However, because of the potential for artifacts and interpretation errors related to intense FDG activity in urinary tract structures (e.g., streak artifacts, confusion of ureteral activity with lymph nodes), various interventions to minimize the amount of FDG in the urinary tract have been employed in different clinics. The current general use of iterative reconstruction algorithms instead of filtered back projection has eliminated most of the image artifacts related to intense urinary tract activity. Nevertheless, in some centers, urinary tract preparation is performed for evaluation of gynecologic cancers. Our procedure is to place a Foley catheter in the urinary bladder, administer intravenous fluids (1,000 to 1,500 mL of 0.9% or 0.45% saline solution to be infused during the course of the study), and intravenous administration of 20 mg furosemide before or after injection of FDG. The Foley catheter should be placed before injection of FDG to minimize radiation exposure to technical or nursing staff. Some investigators prefer the use of continuous bladder irrigation with a double-lumen catheter for the duration of the study.
For PET/CT, the administration of oral contrast agents is helpful for delineating bowel loops and makes image interpretation easier. There is no consensus regarding the need for administration of intravenous contrast agents. PET imaging is delayed for 1 hour after injection of FDG improves the sensitivity for detection of nodal metastasis in cervical cancer. Serum glucose levels are maintained at about 100 mg/dL, and studies are deferred if blood glucose concentration exceeds 200 mg/dL.
Cervical Cancer
Worldwide, cervical cancer is the second most common cause of cancer-related deaths in women. Although less common in the United States, cervical cancer is still expected to account for approximately 11,070 new cervical cancer diagnoses and 3,870 deaths in 2008 (3). Squamous cell carcinomas represent over 90% of cervical cancers and originate in the surface epithelium of the cervix; adenocarcinomas represent approximately 5% to 9% of cervical cancers and originate in the cervical glandular tissue. Adenosquamous carcinoma is relatively infrequent and represents about 2% to 5% of all cervical carcinomas. Rare cervical sarcomas and small cell carcinomas account for the remainder.
Staging
Cervical cancer typically disseminates in a predictable fashion, with initial spread to local structures and regional lymphatics and later hematogenous spread to distant organs, such as bone, brain, liver, and lung. The pattern of nodal metastasis is also predictable: tumor spreads
from the primary lesion sequentially to pelvic lymph nodes, para-aortic lymph nodes, and supraclavicular lymph nodes. Cervical cancer is staged clinically based on the Federation Internationale de Gynecologie et d’Obstetrique (FIGO) staging system. Involvement of pelvic or para-aortic lymph node does not alter the FIGO clinical stage of disease, but indicates a worse prognosis (4,5,6,7) and may have an important impact on therapy. Because of limitations of conventional radiological techniques for evaluating lymph nodes, surgical assessment of para-aortic nodes is considered the gold standard. However, exploratory laparotomy and nodal dissection has not had a demonstrable impact on survival. Moreover, because of the morbidity associated with surgical staging, this procedure is not widely used; thus, the search for an accurate noninvasive staging method is an ongoing process.
from the primary lesion sequentially to pelvic lymph nodes, para-aortic lymph nodes, and supraclavicular lymph nodes. Cervical cancer is staged clinically based on the Federation Internationale de Gynecologie et d’Obstetrique (FIGO) staging system. Involvement of pelvic or para-aortic lymph node does not alter the FIGO clinical stage of disease, but indicates a worse prognosis (4,5,6,7) and may have an important impact on therapy. Because of limitations of conventional radiological techniques for evaluating lymph nodes, surgical assessment of para-aortic nodes is considered the gold standard. However, exploratory laparotomy and nodal dissection has not had a demonstrable impact on survival. Moreover, because of the morbidity associated with surgical staging, this procedure is not widely used; thus, the search for an accurate noninvasive staging method is an ongoing process.
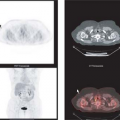
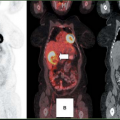
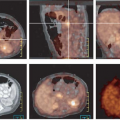
FDG PET appears to be well suited for imaging of cervical carcinoma. Most primary tumors, except for very small lesions, are readily seen on PET images and exhibit intense FDG uptake. In most series, primary squamous cell carcinoma and adenocarcinoma have similar FDG avidity. However, because of its relatively poor spatial resolution and inability to assess parametrial invasion or involvement of adjacent organs reliably, FDG PET is of limited value for staging of the primary tumor.
A number of studies have shown that FDG PET is superior to conventional imaging methods for detecting metastatic disease, particularly lymph node metastasis (8,9). Havrilesky et al. (10) recently reported a systematic review of the published literature up through 2003. They included only those studies involving 12 or more subjects who had PET performed with a dedicated scanner with specified resolution, and with clinical follow-up of 6 months or more or histopathology as the reference standards. In patients with newly diagnosed cervical cancer, the pooled sensitivity of PET was 79% (95% confidence interval [CI], 65% to 90%), and the pooled specificity was 99% (95% CI, 96% to 99%) for detection of pelvic lymph nodes metastasis (9,11,12,13). Two studies were identified that compared PET to magnetic resonance imaging (MRI) and CT (7,8). MRI had a pooled sensitivity of 72% (95% CI, 53% to 87%) and pooled specificity of 96% (95% CI, 92% to 98%), whereas CT had a pooled sensitivity of 47% (95% CI, 21% to 73%) (there were insufficient data to calculate a pooled specificity).
In four prospective studies in which histology after para-aortic lymphadenectomy was used as the reference standard, the pooled sensitivity of PET for the detection of para-aortic nodal metastasis was 84% (95% CI, 68% to 94%) and the pooled specificity was 95% (95% CI, 89% to 98%) (9,11,12,14). In three of these studies, the inclusion criteria for study entry included a negative CT or MRI of the abdomen (10,12,13). Thus, the accuracy of conventional imaging could not be calculated. Reinhardt et al. (9) did not require a negative abdominal imaging study prior to surgery. The sensitivity and specificity of MRI in the 12 patients who underwent aortic node sampling were 67% and 100%, respectively.
False-negative results for detection of nodal metastasis are chiefly related to the limited resolution of PET and, thus, its inability to detect microscopic disease and small macroscopic tumor deposits. In a recent study that evaluated the sensitivity of FDG PET by comparison with surgical lymphadenectomy in patients with early stage cervical cancer the mean size of tumor deposits was larger in PET-positive pelvic nodes (15.2 mm; range 2 to 35 mm) than in PET-negative nodes (7.3 mm; range 0.3 to 20 mm) (15). False-positive results are most likely related to uptake of FDG in hyperplastic nodes or misinterpretation of physiologic activity in bowel or the urinary tract as nodal metastasis. Sironi et al. (16) demonstrated similar findings in their prospective study of FDG PET/CT before radical hysterectomy.
Grigsby et al. (17) have shown that FDG PET is superior to CT and lymphangiography in showing unsuspected sites of metastasis in pelvic lymph nodes, extrapelvic lymph nodes, and visceral organs in patients with newly diagnosed advanced cervical cancer. FDG PET showed abnormalities consistent with metastasis more often than did CT in pelvic lymph nodes (67% vs. 20%) and in para-aortic lymph nodes (21% vs. 7%). PET also showed disease in supraclavicular lymph nodes in 8% (18). These initial results have been sustained in subsequent evaluations of data from a prospective registry that now includes over 400 patients (19).
The role of PET/CT in staging of cervical cancer needs to be determined. The literature currently contains limited data on the use of PET/CT in cervical cancer; however, it is expected that PET/CT image fusion will allow for easier distinction of pathologic and physiologic tracer uptake and, thus, improve the accuracy of image interpretation (20).
Based on the results in the literature to date, the U.S. Center for Medicare and Medicaid Services in January 2005 approved coverage for use of FDG PET in initial staging of patients with cervical cancer who have no evidence of extrapelvic metastatic disease on CT or MRI (21).
Prognosis
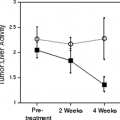
Several prognostic factors have been identified for patients with carcinoma of the cervix. These include patient age, tumor histology, tumor stage, tumor size, lymph node metastasis, and tumor hypoxia (22,23). In a study of 101 patients with newly diagnosed cervical cancer, Grigsby et al. (18) demonstrated that the lymph node status determined by FDG PET is the most significant pretreatment independent predictor of progression-free and overall survival in patients with cervical cancer. The 2-year, disease-free survival was better predicted by PET evidence of lymph node involvement than by CT findings. Based on the imaging findings in the pelvic lymph nodes, the 2-year, disease-free survival was 84% for CT–/PET– patients, 64% for CT-/PET+ patients, and 48% for CT+/PET+ patients (P = .05). Based on the imaging findings in the para-aortic nodes, the 2-year, disease-free survival was 78% in CT–/PET– patients, 31% for CT-/PET+ patients, and 14% for CT+/PET+ patients (P ≤.0001). No patients with PET+ supraclavicular lymph nodes survived 2 years. The PET-determined status of the para- aortic nodes was the strongest predictor of survival in a multivariate logistic regression analysis. These results suggest an opportunity to cure patients with para-aortic nodal metastasis defined by PET, but were not otherwise detected by CT. A recent review of data from 256 patients in the author’s registry found that the extent of lymph node involvement is inversely correlated with survival (19). It was also found that FDG PET demonstrated metastatic involvement in the left supraclavicular lymph nodes in 8% of this patient population (24). This finding had a positive predictive value of 100% and indicates a dismal prognosis, despite aggressive therapy. Similarly, it was found that the cause-specific survival for patients with FIGO stage IIIb carcinoma is highly dependent on the extent of lymph node metastasis demonstrated by whole-body FDG PET at initial presentation (25). The 3-year estimates of cause-specific survival were 73% for those with no lymph node metastasis, 58% for those with only pelvic lymph node metastasis, 29% for those with pelvic and para-aortic lymph node metastasis, and 0% for those with pelvic, para-aortic, and supraclavicular lymph node metastasis (P = .0005).
Extent of regional lymph node metastasis was also found by Unger et al. (26) to be a significant prognostic factor.
Extent of regional lymph node metastasis was also found by Unger et al. (26) to be a significant prognostic factor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree