Infection with certain types of HPV, particularly HPV 16 and 18
Early age at first sexual intercourse
Multiple sexual partners
Genital warts
Immunosuppression
HIV-positive status
Smoking
Long-term use of oral contraceptives
3.3 Anatomy
The cervix is narrower and more cylindrical than the uterine corpus and bulges into the vagina. The portion projecting into the vagina is referred to as the portio vaginalis or ectocervix. The external os (the opening between the cervix and the vagina) is marked histologically by the squamocolumnar junction. The internal os (the opening between the cervix and uterine isthmus) is less clearly demarcated, and it is indicated histologically by the change from cervical fibrous stroma and endocervical mucosa to the mixed histology of the isthmus, which is intermediate between uterine and cervical tissue. The internal os is delineated anatomically by narrowing of the uterine contour and by the entry level of the uterine vessels.
The cervix is supported by multiple ligaments: anteriorly (pubocervical and paired uterovesical ligaments); posteriorly (paired uterosacral ligaments); laterally (paired cardinal ligaments forming the parametria—cellular connective tissue). The parametrium is found adjacent to the lateral margins of the uterus, where the peritoneum reflects to form the broad ligaments. The uterine vessels pass through the lateral parametrium to reach the uterus at the level of the internal os. Parametrial tissue also contains the ureters, as well as many efferent lymphatics.
3.4 Diagnosis
Presentation
In developed countries most cases of cervical carcinoma are diagnosed in asymptomatic patients due to routine screening with a Pap smear. Symptomatic patients present with abnormal vaginal bleeding and occasional vaginal discomfort or malodorous discharge. In patients with advanced disease, symptoms may include flank pain due to hydronephrosis, hematuria due to bladder invasion, rectal bleeding, or other gastrointestinal symptoms.
Histology
Cervical cancer arises from the squamocolumnar junction, which migrates over the years from the ectocervix in young women into the endocervical canal in older women. Therefore, exophytic tumors are typical for young women whereas endophytic tumors are more characteristic for older women.
Squamous cell carcinoma is the most common histologic subtype, responsible for 85 % of primary cervical cancers. Adenocarcinomas account for about 10–12 % of cases, but their incidence is on the rise in more developed countries. It is explained by the fact that adenocarcinoma in situ (the precursor lesion) is detected much less efficiently by Pap smear screening than preinvasive squamous lesions (squamous dysplasia and cervical intraepithelial neoplasia [CIN]). Clear cell carcinoma is a rare subtype of adenocarcinoma accounting for about 5 % of adenocarcinomas of the cervix. In the past, many cases were associated with in-utero exposure to diethylstilbestrol [6]. However, since its use in pregnancy was banned in 1971, the number of cases associated with this drug has diminished. Other uncommon histologic subtypes are adenosquamous carcinomas and small-cell carcinomas. Histologic types of carcinoma found in cervix (World Health Organization [WHO] classification) are summarized in Table 3.2.
Table 3.2
Histological types of cervical cancer (WHO)
Frequency (%) | |
|---|---|
Squamous cell carcinoma Keratinizing Nonkeratinizing Spindle cell carcinoma | 85 |
Adenocarcinoma Endocervical type Variant: adenoma malignum (minimal deviation carcinoma) Variant: villoglandular papillary adenocarcinoma Endometrioid adenocarcinoma Clear cell adenocarcinoma Serous adenocarcinoma Mesonephric adenocarcinoma Intestinal type (signet ring) adenocarcinoma Other epithelial tumors Adenosquamous carcinoma Adenoid cystic carcinoma Small cell carcinoma Undifferentiated carcinoma Metastatic tumors (breast, ovary, colon, lung, and direct spread of endometrial carcinoma) | 10–12 |
3.5 Staging
Clinical Staging
Once the tissue diagnosis of invasive cervical carcinoma is established, the patient is staged. The International Federation of Gynecology and Obstetrics (FIGO) system, last revised in 2009, is the most widely used staging system for cervical carcinoma (Table 3.3) [7]. The FIGO staging of cervical carcinoma is clinical and does not rely on either surgical or pathologic findings. This allows uniformity of staging for all patients worldwide, which is of particular importance as cervical carcinoma is most prevalent in countries where surgical and diagnostic resources are limited.
For small early-stage tumors (stage IA and IB1), stage is assigned after measurement of the depth and width of tumor invasion on cone biopsy, pelvic examination for clinically assessment of tumor size, or both. For more advanced tumors, pelvic examination under anesthesia is sometimes necessary for the parametrial assessment. Additional tests permitted for FIGO staging are summarized in Table 3.4 and are restricted to imaging modalities available in most countries.
It is well known that clinical staging of cervical carcinoma is associated with significant inaccuracies compared to surgical staging, with an error rate up to 32 % in patients with stage IB disease and up to 65 % in patients with stage III disease [8]. The main difficulty is accurate clinical estimation of the tumor size in craniocaudal plane, assessment of parametrial and pelvic side wall invasion, and evaluation of lymph node metastases.
Although revised FIGO staging system does not allow the results of CT, MRI, or positron-emission tomography (PET or PET CT) to influence the clinical stage, the committee does encourage the use of these imaging techniques, if available, to more accurately assess important prognostic factors as tumor size, parametrial and pelvic side wall invasion, adjacent organ invasion, and lymph node status [7, 9, 10].
Stage I | The carcinoma is strictly confined to the cervix (extension to the corpus would be disregarded) |
IA | Invasive carcinoma, which can be diagnosed only by microscopy, with deepest invasion ≤5 mm and the largest extension ≥7 mm |
IA1 | Measured stromal invasion of ≤3 mm in depth and extension of ≤7 mm |
IA2 | Measured stromal invasion of >3 mm and not >5 mm with an extension of not >7 mm |
IB | Clinically visible lesions limited to the cervix uteri or preclinical cancers greater than stage IA |
IB1 | Clinically visible lesion ≤4 cm in greatest dimension |
IB2 | Clinically visible lesion >4 cm in greatest dimension |
Stage II | Cervical carcinoma invades beyond the uterus but not to the pelvic wall or to the lower third of the vagina |
IIA | Without parametrial invasion |
IIA1 | Clinically visible lesion ≤4 cm in greatest dimension |
IIA2 | Clinically visible lesion >4 cm in greatest dimension |
IIB | With obvious parametrial invasion |
Stage III | The tumour extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or non-functioning kidney |
IIIA | Tumour involves lower third of the vagina, with no extension to the pelvic wall |
IIIB | Extension to the pelvic wall and/or hydronephrosis or nonfunctioning kidney |
Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to Stage IV |
IVA | Spread of the growth to adjacent organs |
IVB | Spread to distant organs |
Table 3.4
Procedures permitted for FIGO staging of cervical cancer
Physical examination | Palpation of lymph nodes |
Vaginal examination | |
Rectovaginal examination with or without anesthesia | |
Radiologic studies | Chest radiograph |
Skeletal radiograph | |
Intravenous pyelogram | |
Barium enema | |
Procedures | Cervical biopsy |
Cervical conization | |
Hysteroscopy | |
Colposcopy | |
Endocervical curettage | |
Cystoscopy | |
Proctoscopy | |
Other studies (not allowed for assignment of clinical staging) | Computed tomography |
Magnetic resonance imaging | |
Positron emission tomography with fluorodeoxyglucose | |
Ultrasonography | |
Bone scanning | |
Lymphangiography | |
Laparoscopy |
3.6 Management
Treatment options are summarized in Table 3.5 [5, 11].
Table 3.5
Treatment algorithm for cervical cancer
Stage | Clinical features | Treatment |
|---|---|---|
IA1 | If desires fertility preservation | Observation after cone biopsy with negative margins |
OR | ||
Simple hysterectomy | ||
OR | ||
If lymphovascular invasion | Radical trachelectomy or radical hysterectomy + pelvic lymphadenectomy | |
IA2 | If desires fertility preservation | Radical trachelectomy + pelvic lymphadenectomy ± para-aortic lymph node sampling |
OR | ||
Radical hysterectomy + pelvic lymphadenectomy ± para-aortic lymph node sampling | ||
OR | ||
Radiotherapy | ||
IB1 and IIA1 | If desires fertility preservation, stage IB1 only and tumor is <2 cm | Radical trachelectomy + pelvic lymphadenectomy ± para-aortic lymph node sampling |
OR | ||
Radical hysterectomy + pelvic lymphadenectomy ± para-aortic lymph node sampling | ||
OR | ||
Radiotherapy | ||
IB2 and IIA2 | Chemoradiotherapy | |
OR | ||
Radical hysterectomy + pelvic lymphadenectomy + para-aortic lymph node sampling | ||
OR | ||
Chemoradiotherapy ± adjuvant hysterectomy | ||
IIB and IIIA | Chemoradiotherapy | |
IVA | Chemoradiotherapy | |
OR | ||
Primary pelvic exenteration | ||
IVB | Palliative chemotherapy | |
OR | ||
Chemoradiotherapy |
3.7 Imaging
Cervical cancer is typically detected clinically (Pap smear and/or physical examination)
Imaging
Evaluation of extent of disease
Radiation therapy planning
Monitoring treatment response
Detection of tumor recurrence
Guidance prior to and during interventional procedures
Ultrasonography (US) | |
Transabdominal US | No role in evaluating local extent; may be used to detect hydronephrosis |
Similar accuracy to MRI for tumor detection and parametrial evaluation | |
Transrectal (TRUS) US | BUT |
Operator dependent | |
Narrow field of view (FOV) yields no information regarding nodal status | |
Computed tomography (CT) | Inferior to MRI in local staging of early-stage cervical cancer |
Limited soft tissue contrast accounts for poor detection of small tumors, parametrial invasion, and early invasion of the rectum and bladder mucosa | |
For detecting parametrial invasion: sensitivity, 17–100 % (average, 64 %); specificity, 50–100 % (average, 81 %) | |
Value of CT increased with higher stage disease | |
Detection of distant metastases and nodal assessment | |
Based on the ACRIN trial results, sensitivity, specificity, and negative predictive value (NPV) for staging FIGO stage IIB or greater tumors: 42, 82, and 84 %, respectively | |
Nodal assessment | |
Low sensitivity as it relies on size criterion alone(>1 cm in short axis)for diagnosis of malignant adenopathy | |
Cannot detect micrometastases | |
Sensitivity, 31–65 %; positive predictive value (PPV), 51–65 %; and NPV, 86–95 % | |
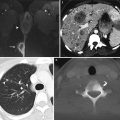
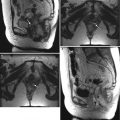
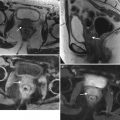
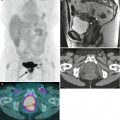
Magnetic resonance imaging (MRI) | Imaging modality of choice for assessment of tumor location (exophytic or endocervical), its size, and presence of invasion into the parametria, pelvic sidewall or adjacent organs |
Complimentary to clinical assessment in staging FIGO stage IB or higher tumors | |
Overall staging accuracy, 75–96 % | |
Superior to clinical examination in assessing tumor size, especially its craniocaudal dimension | |
Superior to CT in detecting parametrial invasion; sensitivity and specificity in evaluating parametrial invasion, 40–57 % and 77–80 %, respectively | |
Neither CT nor MRI are accurate for evaluating cervical stroma | |
MRI is mandatory in patients considered for fertility sparing radical trachelectomy | |
MRI performs similar to CT in nodal assessment | |
Also relies on size criteria for assessing lymph nodes | |
Sensitivity, 30–73 % and specificity, 93–95 %, respectively | |
Positron emission tomography (PET) and PET/CT | Imaging modality of choice for assessment of pelvic and extrapelvic lymph nodes and distant organ involvement, particularly in patients with advanced cervical cancer (i.e., FIGO stage IIB or higher) |
Sensitivity, 58–72 %; specificity, 93–95 %; accuracy, 85–99 % in detection of nodal metastases; these values are higher than those for MRI and CT | |
Value of FDG PET in early stage disease (FIGO I to IIA) is questionable | |
Low sensitivities for detection of nodal metastases, 25–73 % | |
Does not replace the need for lymphadenectomy for nodal assessment | |
FDG activity may be important in predicting outcome | |
Detection of persistent or recurrent disease after chemoradiation | |
Future studies may use the best of both techniques with MRI/PET fusion imaging |
Imaging Techniques
Patient position | Supine |
Prone | |
Maybe use in CT-guided biopsy or drainage | |
Imaging | Oral contrast medium |
750–1,000 mL diluted oral contrast 2 h prior to examination | |
Intravenous contrast medium | |
100–150 mL iodinated contrast medium | |
Injection rate: 2–3 mL/s | |
70–120 s delay after IV contrast administration | |
5 mm collimation (thinner for 3D or small lesion characterization) |
Patient preparation | To limit artifacts due to small bowel peristalsis |
Fasting for 4–6 h before MRI examination | |
Optional: use of antiperistaltic agents (hyoscine butyl bromide or glucagon) | |
Optional: vaginal opacification with sonographic gel | |
Helpful in cases of suspected cervical tumor extension into the vagina, especially posterior fornix | |
Empty bladder | |
Coil selection | Image supine using pelvic surface array multichannel coil |
Improves signal-to-noise ratio, increases resolution, and decreases imaging time | |
Endoluminal coils: rarely used | |
Advantages | |
High-resolution images of small tumors | |
Aid in detection of early parametrial extension | |
Disadvantages | |
Extra cost | |
Patient discomfort | |
Small FOV which is inadequate for evaluation of large tumors and their extrauterine extent | |
Not an issue if combined with the use of the pelvic surface array coil | |
Imaging sequences and planes | Transverse T1-WI using large FOV that includes entire pelvis |
Evaluation of lymph nodes and pelvic bones | |
Small FOV T2-WI without fat suppression in at least two orthogonal planes such as sagittal and oblique: | |
Sagittal plane | |
Distance to the internal cervical os and tumor extension into the lower uterine segment (if fertility sparing surgery is desired) | |
Evaluation of tumor invasion into the urinary bladder and vagina | |
Oblique planes | |
Transverse oblique (perpendicular to the long axis of the cervical canal) and/or coronal oblique (parallel to the long axis of the cervical canal) | |
Crucial for accurate evaluation of parametria | |
Preferred imaging parameters | |
FOV 20–25 cm, slice thickness (3–4 mm/0.4 mm skip), matrix 512 × 512 mm | |
Optional: | |
Large FOV coronal T2-WI | |
Assessment of kidneys and ureters in patients with advanced stage disease (FIGO stage III-IV) | |
Dynamic contrast-enhanced sagittal 3D GRE T1-WI | |
Can be useful in detection of small cervical tumors, assessment of tumor response, and distinguishing radiation-induced fibrosis from residual or recurrent tumor | |
Precontrast and 4 postcontrast sequences at 1 min intervals | |
DWI –MR (b values: 0, 500–1,000 s/mm2) | |
Potentially useful in tumor detection/ staging and assessment of tumor response |
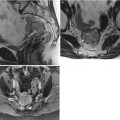
Normal Anatomy
Table 3.9




Normal zonal anatomy of the cervix on T2-WI [17]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree