Fig. 1
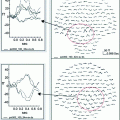
An absence seizure recorded during hyperventilation. Top 8 s of MEG data maxfiltered using tSSS with motion correction, displayed on left frontal channels showing seizure onset during hyperventilation. The sources of MEG activity at seizure onset was calculated using a joint minimum-variance beamformer and spike-detection algorithm, a procedure called SAM(g2) (Kirsch et al. 2006). The beamformer output at a target location has the same temporal resolution as the recorded MEG signals, and is therefore often referred to as a virtual electrode (Robinson and Vrba 1999) and can be seen as a morphologic characterization of the regional electrical activity. Spike-like activity is identified from the estimated source data in terms of excess kurtosis. Two virtual electrodes yielded by this analysis (VE1 and VE2) are displayed beneath. Bottom Source localization of the two virtual electrodes showing regions of high kurtosis bilaterally in the frontal lobe is shown
To make the best of head movement-compensation systems during recordings, it is important to ensure that the coils remain within the MEG sensor-space and as close to the sensors as possible. This can be challenging when working with the youngest children. When inserted as far as possible into the MEG system, children may find that their vision is obscured by the front of the helmet, and have a tendency to lean forwards and downwards, resting their upper forehead against the forward edge of the helmet, in order to see out. In doing so, they may bring any coils been placed on the forehead outside of the sensor space, making head position estimation, and therefore correction, impossible. Encouraging the child to ‘keep their chin up’ is therefore important; future modifications to proprietary head motion correction systems should aim to account for this.
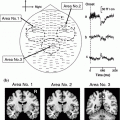
The second implication of recording from smaller heads in addition to an increased distance is a reduction in sensor coverage of the anterior temporal lobes, and this also provides another reason to encourage a school-age patient to lean back in the MEG helmet. The anterior temporal lobes lie only a few centimeters behind the eyes, and are easily brought outside the sensors if the head is pitched forward and down. Figure 2 shows examples of auditory N1 field patterns recorded from an adult, and from two children using a CTF MEG system with axial gradiometers, illustrating this loss of signal from the smaller heads. The source of the auditory N1 is in the planum temporale, just posterior to the Heschl’s gyrus, yet half of the field pattern is lost. Clearly, depending on its orientation, temporal-lobe epileptiform activity could be lost in the same way and this problem may at least in part explain the reported relative low sensitivity in the mesial temporal lobe (e.g. Agirre-Arrizubieta et al. 2009; Leijten et al. 2003).
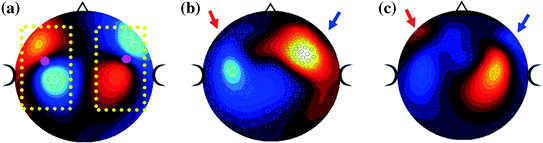

Fig. 2
Three field patterns from a CTF 275-channel MEG system, for evoked responses elicited by an auditory click. The field patterns reflect the activity at about 100-ms after presentation, for the response known as the N1m which originates in planum temporale, just posterior to primary auditory cortex and about mid-way along the temporal lobe. ‘a’ shows data for a typical adult, with red and blue mirror-image dipolar field patterns in the left and right hemisphere, highlighted by the yellow box. In ‘b’, the recording is from a child and the anterior portion of the field pattern in each hemisphere is lost (indicated by the red and blue arrows). ‘c’ shows another child recording, where the field patterns are also incomplete. These field patterns illustrate the relative lack of sensitivity to temporal lobe sources in child patients, compared to adults, when recorded in an adult MEG system
The most critical implication of recording from smaller heads is that if the brain surface is further from the sensors, the recorded signal will be smaller. Acknowledging that overall head growth incorporates changes in the size of the cranium as well as of brain-size, we can safely assume that the cerebral cortex is several millimeters, often centimeters, further from the MEG sensors in our youngest patients compared to the adults for whom the system was designed. This can affect signal-to-noise quite considerably (Gaetz et al. 2008). Simulated data have previously shown that whilst superficial MEG sources have in general a high detection probability, the maximum detection probability starts to fall off very dramatically when the minimum source-sensor distance is larger than 6.5 cm (Hillebrand and Barnes 2002). Thus, signal from the anterior and inferior frontal and temporal lobes are sub-optimal in adult heads and may become undetectable in smaller children.
2.2 Stature
Children’s smaller stature poses some additional problems for successful MEG recordings, including the suitability of seating arrangements, and the ability to fully insert the child’s head into the MEG helmet. Recording while seated, rather than supine, has many benefits, most importantly that the child can feel much more at ease. The child can see the room around them and be reassured by the ease of making eye contact with a parent or other adult figure accompanying them in the magnetically shielded room. When the patient is seated, it is also much easier for them to use response-devices such as joysticks or button-boxes, which can be placed on a table in front of them and easily visible.
However, like MEG helmet sizes, the adjustable chairs supplied with MEG systems were designed with adult patients in mind. With both the MEG systems we have used at Aston, limits to the maximum height of the seat meant that our younger school-age patients, with the seat at maximum height, are not fully inserted into the MEG helmet. The addition of cushions is not ideal, because cushions have a tendency to compress with time, so the child sinks below the sensors during the recording. Booster seats intended for use in cars have offered some success, but are not always comfortable, because we are using them with children much older than those for whom the seats were designed. Improved seating arrangements should be easy to achieve and a cost-effective priority for MEG manufacturers seeking to optimize their systems for use with children.
Whether seated or supine, children of very small stature may also be impossible to insert fully into the helmet for anatomical reasons (Fig. 3). The depth of the helmet, around 22—24 cm (depending on the manufacturer) may be longer than the distance between a very young child’s shoulders and the crown. This means that, as the child’s head is positioned within the helmet, their shoulders will come into contact with the lower limits of the helmet before their head reaches the top of the helmet, leaving a gap between the top of their head and the upper MEG sensors (see Fig. 3a). Our calculations based on population-level anthropometric data (Snyder et al. 1975) suggest that this is a problem even for the shallowest MEG helmets (22 cm) for typically developing children between about 4 and 6 years of age (Fig. 3b).
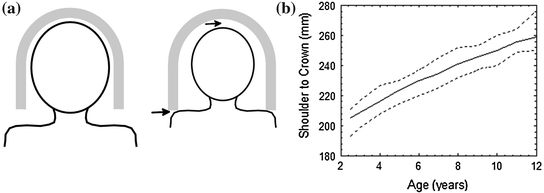

Fig. 3
Panel A Illustration of how stature affects insertion into the MEG helmet. An adult, on the left panel, can be fully inserted into the helmet; but for a child, the shoulders touch the lower edge of the helmet before the crown of the head reaches the top (arrows). Panel B The distance from shoulder to crown in TD children, as a function of age: data from Snyder et al. (1975). The Elekta MEG system helmet has a depth of about 22 cm and the CTF of about 23.5 cm. Children with a smaller shoulder-crown distance than this depth will not be fully inserted into the helmet.
A very important observation, in relation to the design of MEG systems and accessories for use with children, is that pediatric patients are smaller than typically developing children—often considerably so. Figure 4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree