Inflammatory brain diseases in childhood are underrecognized and lead to devastating yet potentially reversible deficits. New-onset neurologic or psychiatric deficits in previously healthy children mandate an evaluation for an underlying inflammatory brain disease. Distinct disease entities, such as central nervous system (CNS) vasculitis, are now being increasingly reported in children. Clinical symptoms, initial laboratory test, and neuroimaging studies help to differentiate between different causes; however, more invasive tests, such as lumbar puncture, conventional angiography, and/or brain biopsy, are usually necessary before the start of treatment. This article focuses on childhood CNS vasculitis.
Key points
- •
Inflammation has to be considered as the underlying pathomechanism in children presenting with newly acquired neurological and/or psychiatric deficit. Inflammation of the cerebral blood vessel walls can solely target the brain and spinal cord and is then termed primary CNS vasculitis. Several underlying conditions have been found to be associated with a secondary CNS vasculitis; a careful diagnostic evaluation for these conditions is mandatory.
- •
Neuroimaging is crucial in guiding the diagnostic evaluation in childhood CNS vasculitis. It identifies characteristic features of distinct CNS vasculitis subtypes. Children with angiography positive CNS vasculitis commonly present with stroke features, have vascular stenoses or other abnormalities and may have evidence of contrast in the thickened, inflamed cerebral vessel wall. In contrast, neuroimaging may demonstrate multiple T2/Flair positive lesions in non-large vessel territories in children with angiography-negative small vessel vasculitis, which is confirmed on brain biopsy. Neuroimaging has a high sensitivity, but lacks specificity for distinct subtypes of childhood inflammatory brain diseases.
- •
Early recognition, rapid diagnostic evaluation including novel imaging strategies such as contrast wall enhancement and timely initiation of targeted immunosuppressive therapy have dramatically improved the outcome of children with primary and secondary CNS vasculitis.
Introduction
Inflammation is an increasingly recognized underlying pathologic condition in children presenting with acquired neurologic deficits. All the individual components of the central nervous system (CNS) and peripheral nervous system can be targets of a dysregulated innate or adaptive immune system. The interaction between the target structure and the specific antibodies or cellular response will determine the clinical phenotype of the disease, including the mode of onset, severity, and long-term evolution. A typical clinical presentation of inflammatory brain disease in children is subacute, often multifocal, with a fluctuating but rapid progressive course, either idiopathic or less frequently in the context of a systemic illness or paraneoplastic process.
Primary inflammatory brain diseases solely affect the brain and/or spinal cord and encompass vasculitis and nonvasculitic diseases, such as demyelination, neuronal antibody mediated inflammation, T-cell mediated diseases, and granulomatous inflammatory brain diseases. Secondary inflammatory brain diseases result when brain inflammation occurs in the context of a systemic disease, such as infections, rheumatic diseases, systemic inflammatory diseases, and other systemic illness or exposures. The diagnosis of inflammatory brain disease is based on a thorough clinical evaluation, including features of systemic inflammatory illnesses; blood and cerebrospinal fluid (CSF) analysis; neuroimaging studies; supportive testing, such as electromyography/nerve conduction studies and electroencephalography; and targeted tests, such as specific antibodies or brain biopsies.
Every child with a newly acquired neurologic deficit (focal or systemic) should be investigated for an underlying inflammatory cause. The differential diagnosis for neuro-inflammatory conditions is very wide and rapidly expanding. This article focuses on childhood CNS vasculitis, whereby the target of inflammation is the blood vessels, and its resultant effects on neurologic functioning.
Introduction
Inflammation is an increasingly recognized underlying pathologic condition in children presenting with acquired neurologic deficits. All the individual components of the central nervous system (CNS) and peripheral nervous system can be targets of a dysregulated innate or adaptive immune system. The interaction between the target structure and the specific antibodies or cellular response will determine the clinical phenotype of the disease, including the mode of onset, severity, and long-term evolution. A typical clinical presentation of inflammatory brain disease in children is subacute, often multifocal, with a fluctuating but rapid progressive course, either idiopathic or less frequently in the context of a systemic illness or paraneoplastic process.
Primary inflammatory brain diseases solely affect the brain and/or spinal cord and encompass vasculitis and nonvasculitic diseases, such as demyelination, neuronal antibody mediated inflammation, T-cell mediated diseases, and granulomatous inflammatory brain diseases. Secondary inflammatory brain diseases result when brain inflammation occurs in the context of a systemic disease, such as infections, rheumatic diseases, systemic inflammatory diseases, and other systemic illness or exposures. The diagnosis of inflammatory brain disease is based on a thorough clinical evaluation, including features of systemic inflammatory illnesses; blood and cerebrospinal fluid (CSF) analysis; neuroimaging studies; supportive testing, such as electromyography/nerve conduction studies and electroencephalography; and targeted tests, such as specific antibodies or brain biopsies.
Every child with a newly acquired neurologic deficit (focal or systemic) should be investigated for an underlying inflammatory cause. The differential diagnosis for neuro-inflammatory conditions is very wide and rapidly expanding. This article focuses on childhood CNS vasculitis, whereby the target of inflammation is the blood vessels, and its resultant effects on neurologic functioning.
Primary CNS vasculitis
Primary angiitis of the central nervous system (PACNS) is the most common cause of severe, acquired neurologic deficits in previously healthy children. PACNS was first described in adults in 1959. Initial cases were almost exclusively diagnosed at autopsy, demonstrating granulomatous inflammation of the cerebral arteries. In 1988, Calabrese and Mallek described 8 new cases and summarized the available literature of PACNS in adults. He coined the term PACNS and proposed diagnostic criteria for adults. These criteria mandate (1) a newly acquired neurologic deficit, (2) angiographic and/or histologic evidence of CNS vasculitis, and (3) the absence of a systemic condition that could explain these findings. The Calabrese criteria were adopted and modified for childhood PACNS (cPACNS), requiring a newly acquired neurologic deficit and/or psychiatric symptom in patients aged 18 years or younger. In the authors’ tertiary care center, cPACNS was the most frequently diagnosed inflammatory brain disease over the past 5 years. The current classification of cPACNS is based on affected cerebral vessel size and disease presentation and natural history. Three subtypes are currently recognized: (1) nonprogressive (NP) large-medium vessel cPACNS (angiography positive), (2) progressive (P), large-medium vessel cPACNS (angiography positive), and (3) small vessel (SV) cPACNS (angiography negative, biopsy positive). The 3 subtypes display distinct presenting symptoms, laboratory findings, disease course, and treatment outcome.
Angiography-Positive NP-cPACNS
Clinical features
Children with NP-cPACNS typically present with sudden-onset focal neurologic deficits and are frequently diagnosed with arterial ischemic stroke. This subtype affects boys more commonly than girls, corresponding to the gender predilection in stroke overall. Focal deficits can include abrupt onset of aphasia, visual disturbance, ataxia, hemiparesis, hemifacial weakness, hemisensory loss, and fine motor skill loss. The presentation can either be hyperacute and acute or sometimes of a stutteringtype. The latter refers to recurrent focal deficits lasting few minutes/hours, eventually progressing to a complete irreversible deficit either within one or multiple vascular territories depending on the extent of the vasculitis. Approximately 10% of children present with additional diffuse focal deficits, such as decreased cognition or behavior change. Overall, headaches are present in 40% of the children with NP-cPACNS. Seizures are not a frequent feature but can be present, particularly in younger children.
Laboratory tests
Systemic inflammatory markers, including C-reactive protein and erythrocyte sedimentation rate (ESR), are frequently normal. The endothelial cell marker von Willebrand Factor antigen has been documented to be elevated in some patients but remains to be studied systematically in this population. In NP-cPACNS, less than 50% of the patients have an elevated protein level or evidence of leukocytosis on CSF analysis. The role of the opening pressure remains uncertain. Thus, the value of seeking inflammatory markers either in blood and CSF seems limited from the diagnostic point of view. The presence of inflammatory abnormalities can certainly help in diagnosis, but their absence does not rule out CNS vessel wall inflammation. Thus, there is a clear need for other diagnostic markers, which could be potentially used to reliably detect CNS inflammation in the absence of systemic signs of inflammation. One such marker, CSF neopterin, which is released by CNS macrophages, appears and merits further research because it has been demonstrated to be elevated in a variety of inflammatory disorders affecting the CNS, although it is not clear whether the elevated levels represent a primary inflammation in the CNS or a secondary inflammatory response to non–immune-mediated brain damage.
The evaluation of potential prothrombotic abnormalities is mandatory in most patients. But the role of thrombophilia testing has not been carefully studied in this specific population, although testing can be potentially beneficial in terms of ascertaining stroke recurrence risk because it is becoming increasingly clear that stroke in children results from a disturbance of multiple risk factors. Abnormal tests do require repeat testing in 10 to 12 weeks to check for persistence of the abnormality.
Neuroimaging features
The mainstay of diagnosis of NP-cPACNS is neuroimaging. Plain and contrast head computed tomography (CT) have limited diagnostic value in children who present with an acute focal neurologic deficit because the differential diagnosis is wide in children in addition to stroke caused by NP-cPACNS. This circumstance necessitates the use of magnetic resonance (MR) imaging for a rapid and accurate diagnosis of NP-cPACNS. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient maps are vital for the diagnosis of acute ischemic stroke, which is essentially the sole presenting feature of NP-cPACNS. Based on the duration of symptoms, other sequences, such as T1, T2, and fluid-attenuated inversion recovery (FLAIR), will show the evolution of radiographic changes. T2 or gradient echo or susceptibility-weighted imaging are important in the acute setting to detect hemorrhage because this can influence the decision to initiate thrombolytic or antithrombotic therapy for the acute stroke. The role of perfusion-weighted imaging, either with CT or with MR imaging, to detect “brain tissue at risk for further ischemia in the presence of a documented thrombus in a major cerebral blood vessel” and inform decisions to initiate thrombolytic therapy has been established in adults but not yet in children. This area of neuroimaging in pediatric stroke deserves further study.
The strokes in NP-cPACNS typically involve the basal ganglia for reasons mentioned later. Cortical and subcortical ischemic infarction can also be seen. The mechanism of stroke is either related to artery-to-artery embolism of hypoperfusion injury distal to an occluded artery. The anterior circulation is more frequently involved than the posterior circulation in terms of stroke location.
Intracranial vascular imaging is mandatory for demonstration of the vascular abnormalities. Time-of-flight MR angiography (TOF-MRA) is typically available in all centers now and is reasonably helpful in the demonstration of vascular abnormalities, but it has the potential to both underestimate as well as overestimate abnormalities in children. Gadolinium-enhanced MRA (Auto-Triggered Elliptic Centric Ordered sequence [ATECO]-MRA) has been used in adults for a much better demonstration of the intracranial blood vessels, although this technique has not been systematically studied in children, mainly because of technical difficulties. CT angiography (CTA) can overcome the artifact-related problems with TOF-MRA in children based on the authors’ experience. However, radiation exposure is always a concern; hence, this technique should be used cautiously in children. Conventional catheter cerebral angiography (CCCA) is the gold standard for demonstrating vasculitis. The authors use this technique at their center in carefully selected cases of CNS vasculitis for diagnosis. The invasive nature of the technique and the lack of easily available skilled pediatric neuroangiographers remain big barriers to its wider applicability. Gadolinium-enhanced contrast study of the affected vascular wall segment is a promising new technique to detect large/medium vessel vasculitis and should be requested. In NP-cPACNS, this sequence may reveal wall thickening and concentric contrast enhancement of the affected segment of the vessel wall. The mechanism of gadolinium uptake by the arterial wall in this condition is poorly understood and is speculated to be a result of wall inflammation, although this has not yet been proven. Vessel wall imaging is a potentially exciting area for prospective studies in CNS vasculitis imaging.
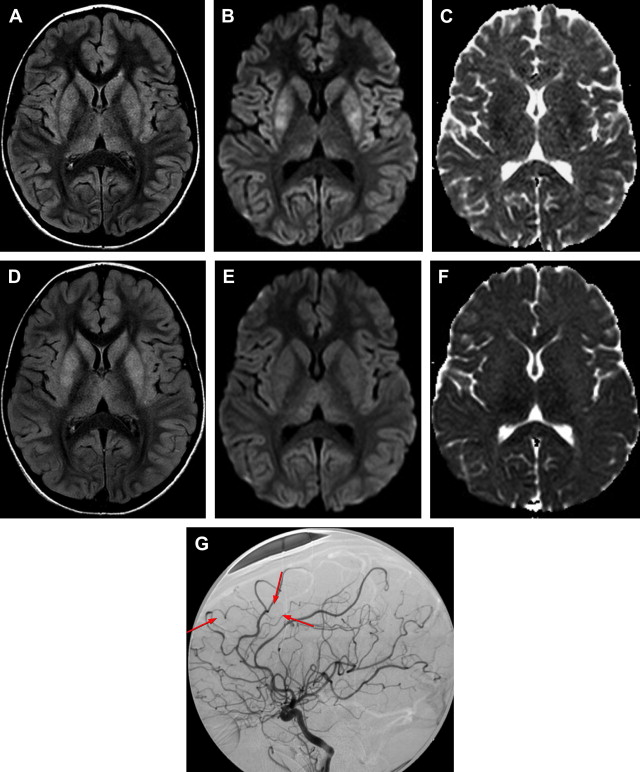
In NP-cPACNS, a variety of vascular abnormalities are seen. These abnormalities include narrowing/stenosis ranging from mild to complete occlusion, irregularity, concentric bands of narrowing ( Fig. 1 ), and vasospasm. Sometimes intraluminal thrombus can be seen either partially or fully occluding the lumen of the affected vessel. The typical location of vasculitis is unilateral; the anterior circulation is predominantly involved, with posterior circulation being far less frequently affected. Isolated posterior circulation involvement is exceedingly rare. In the anterior circulation, selective involvement of the distal internal carotid artery (ICA), proximal segments of the anterior (ACA) and/or middle (MCA) cerebral arteries in varying combinations is seen, whereas involvement of the more smaller-sized arteries is rather uncommon. The site of vascular involvement seems typical and explains the higher frequency of basal ganglia strokes in this condition because the lenticulostriate arteries, the sole source of blood supply for the basal ganglia, arise from the proximal segments of the MCA and ACA.
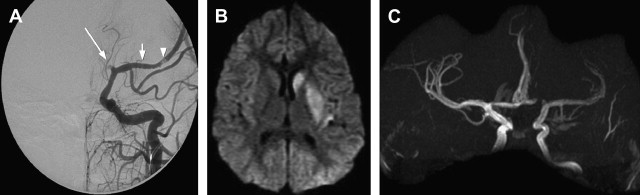
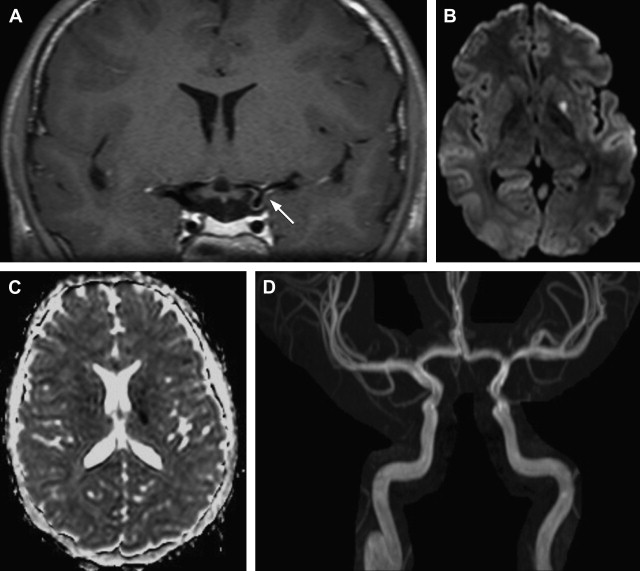
The radiological features of NP-cPACNS are quite typical; in the presence of concordant clinical features, the diagnosis is relatively easy. Similar vascular appearances, however, could potentially occur with other vasculopathies, such as intracranial vessel dissection (although extracranial dissection is more common in children), unilateral moyamoya, and a thrombus arising from a downstream vessel or heart and lodging in the affected vessel. In the authors’ experience, these seem to be rare and, when suspected, do need specific investigations for clarification. For instance, to rule out dissection, CCCA and T1 fat-saturated sequence on MR imaging (to screen for eccentric wall hematoma) and T1 wall imaging with contrast (concentric gadolinium uptake by the affected wall helps in the diagnosis) can be helpful ( Fig. 2 ). For moyamoya, CCCA remains the gold standard for staging and, in particular, detecting hypertrophied basal ganglia perforators, the moyamoya vessels. For detecting thromboembolism from a downstream vascular structure, MRA of the neck vessels and cardiac echo can be helpful. In a small proportion of children at the authors’ center, NP-cPACNS cases with normal MRA and CCCA at diagnosis have been encountered, which on serial follow-up imaging (4–6 weeks, 3 months) were found to have abnormalities, such as stenosis/narrowing of the expected large vessels in the anterior circulation. This finding shows that a child with acute basal ganglia stroke caused by NP-cPACNS may have no abnormalities on vascular imaging; therefore, close, careful follow-up is essential over the initial 6 months or so to prove or disprove the presence of NP-cPACNS.
Etiopathogenesis
The pathophysiology and cause of NP-cPACNS is still not well understood. NP-cPACNS in its current form has been labeled as transient cerebral arteriopathy (TCA) coined by Chabrier and colleagues and more recently as “unilateral focal cerebral arteriopathy of childhood (FCA)” by Bernard and colleagues. It is generally accepted that a subset of NP-cPACNS aka TCA aka FCA are related to varicella (postvaricella angiopathy, PVA) because exactly similar clinicoradiological appearances have been reported in children with basal ganglia strokes following a varicella infection in the preceding 12 months of presentation. This finding raised the hypothesis that PVA is an inflammatory vasculopathy affecting the large vessels of the anterior circulation and mediated by either reactivation of the virus in the nerves (trigeminal system) innervating the circle of Willis causing invasion of the vessels by the virus or triggering a lymphocytic infiltration of the affected vessels. There is some pathologic data to support this hypothesis. But whether all cases of TCA are postinfectious or inflammatory is still debated. Hence, Braun and colleagues came up with the term focal unilateral intracranial vasculopathy because their argument, also supported by others, was that there could be some children in whom TCA might not necessarily have an inflammatory basis; hence, it would be more appropriate to simply give a descriptive term to the radiological picture of the typical involvement of the distal ICA and proximal ACA and MCA in these children. It is also argued that the term TCA is misleading because the progression of vascular abnormalities can occur after diagnosis, albeit over the short-term duration of 3 to 6 months. Thus, it is clear that several aspects of the pathophysiology, cause, and pathology of NP-cPACNS still have not been well elucidated, mainly because of the lack of consistent pathologic data because specimen of blood vessels are understandably difficult to acquire. However, as the current thinking stands, most centers consider NP-cPACNS as a monophasic vasculopathy of likely inflammatory origin that is progressive only in the short-term without evolution into chronicity.
Management
A 3-pronged approach is recommended for the treatment of NP-cPACNS: (1) neuroprotection for the acute ischemic stroke, (2) antithrombotic therapy to treat/prevent thrombus formation in the affected vessel, and (3) immunomodulation for vessel wall inflammation.
Neuroprotection for any child with acute ischemic stroke caused by NP-cPACNS is mandatory. Maintenance of normothermia, normovolemia, normotension, and normoglycemia is vital to protect the “brain tissue at risk” beyond the infracted core of the stroke. The prevention of increases in intracranial pressure, particularly with large MCA strokes, is important. Decompressive craniectomy may need consideration in some children if malignant cerebral edema is imminent. Prompt management of seizures is also recommended because they can increase the cerebral metabolic demand and have the potential to recruit larger areas of the brain in the infracted zone. Prophylactic anticonvulsants are, however, controversial. A low threshold to electroencephalogram (EEG) and, in selected cases, continuous EEG monitoring does need consideration. Overall, the management of children in the intensive care unit can be beneficial.
Children with NP-cPACNS are typically commenced on antithrombotic therapy at diagnosis, although regimens vary between centers. The rationale behind starting antithrombotics in the presence of a preexisting thrombus in the affected vessel wall is to prevent clot propagation and allow the body’s fibrinolytic system to dissolve the clot. In certain instances, thrombolytic therapy (tissue plasminogen activator [tPA]) has been potentially considered in teenagers with NP-cPACNS who have a confirmed stroke with documented intraluminal thrombus, provided they are within the time window and there are no contraindications to give tPA. It must, however, be remembered that safety of tPA in the pediatric age group has not yet been well established. In the absence of a documented luminal thrombus, the rationale behind giving antithrombotic agents is to prevent clot formation in the inflamed vessel because inflammation causes narrowing of vessel lumen and triggers both the coagulation cascade as well as activates platelets increasing the risk of thrombosis. The choice between anticoagulants and antiplatelets is a matter of debate because there are no head-to-head comparative trials in pediatric stroke. Consensus-based treatment guidelines have still not adopted a completely unified approach to antithrombotic therapy in childhood stroke. As a result, treatment is usually based on the preferences of individual centers and physicians. Frequently, anticoagulants, mainly heparin derivatives, are started in the acute setting, particularly in children with a high degree of vascular stenosis followed by long-term, low-dose aspirin for secondary stroke prevention. However, the duration of the treatment for heparin and aspirin, both in the short term and/or long term, varies considerably between centers and reflects the lack of prospective clinical studies in pediatric stroke. Most centers will, however, continue children on some form of antithrombotic therapy for at least 1 to 2 years after the initial stroke provided all risk factors, including vascular risk factors, are no longer active. In the presence of persisting risk factors of recurrent events and significant vascular stenosis, children with NP-cPACNS commonly receive antithrombotic agents, particularly aspirin, for several years.
Immunomodulation therapy with corticosteroids for 3 months remains controversial.
Outcome
Nonprogression of vascular changes is usually confirmed on repeat vascular imaging at 3 months, establishing no evidence of involvement of new vascular beds and resolution of contrast enhancement in the vascular wall in steroid-treated patients. Recurrent ischemic events are seen in 30% to 60% of children. The long-term outcome remains to be systematically studied; it seems to be closely related to the location and extent of the ischemic lesion, stroke recurrence, and possibly the use of corticosteroids. Early comprehensive rehabilitation, as practiced in adult stroke care models, has not been systematically performed or studied in this population; however, it seems to have striking benefits.
Angiography-Positive P-cPACNS
Children with P-cPACNS can commonly present with both focal and diffuse neurologic deficits. Interestingly, both angiography-positive CNS vasculitis subtypes, NP-cPACNS and P-cPACNS, predominately affect boys. Children with P-cPACNS are commonly diagnosed when they develop focal deficits, including hemisensory loss or fine motor skill deficits. In addition, difficulty in concentration, cognitive dysfunction, and mood and personality changes are present in these patients. These diffuse deficits develop insidiously. Correspondingly, the time from onset of any symptoms to diagnosis is frequently longer in patients with P-cPACNS compared with patients with NP-cPACNS. Headaches are the leading clinical symptom and present in 95% of patients with P-cPACNS. Systemic underlying conditions have to be carefully looked for and excluded because the clinical and imaging pattern of P-cPACNS is frequently found in angiography-positive, secondary CNS vasculitis of childhood (see later discussion).
Children with P-cPACNS may have mild to moderately increased inflammatory markers; however, inflammatory markers and CSF analysis are not discriminative. A normal CSF cell count or a normal ESR does not exclude an angiography-positive CNS vasculitis. Required MR imaging sequences are identical to those performed in suspected NP-cPACNS. In P-cPACNS, parenchymal lesions on MR imaging can be ischemic and/or inflammatory and are commonly present in more than one vascular territory. One in 4 children has bilateral MR imaging lesions, which are more frequently asymmetric in appearance. The angiography characteristically demonstrates vasculitis of proximal and distal segments of the cerebral arteries ( Fig. 3 ), typically involving multiple vascular beds. Different degrees of gadolinium contrast enhancement can be found in affected vessel wall segments. The anterior circulation is more commonly affected; isolated posterior circulation vasculitis is less common. Conventional angiography provides additional information about the length and degree of stenosis potentially impacting in antithrombotic treatment choices. It also visualizes collateral blood flow into the affected brain tissue identifying additional brain at risk.