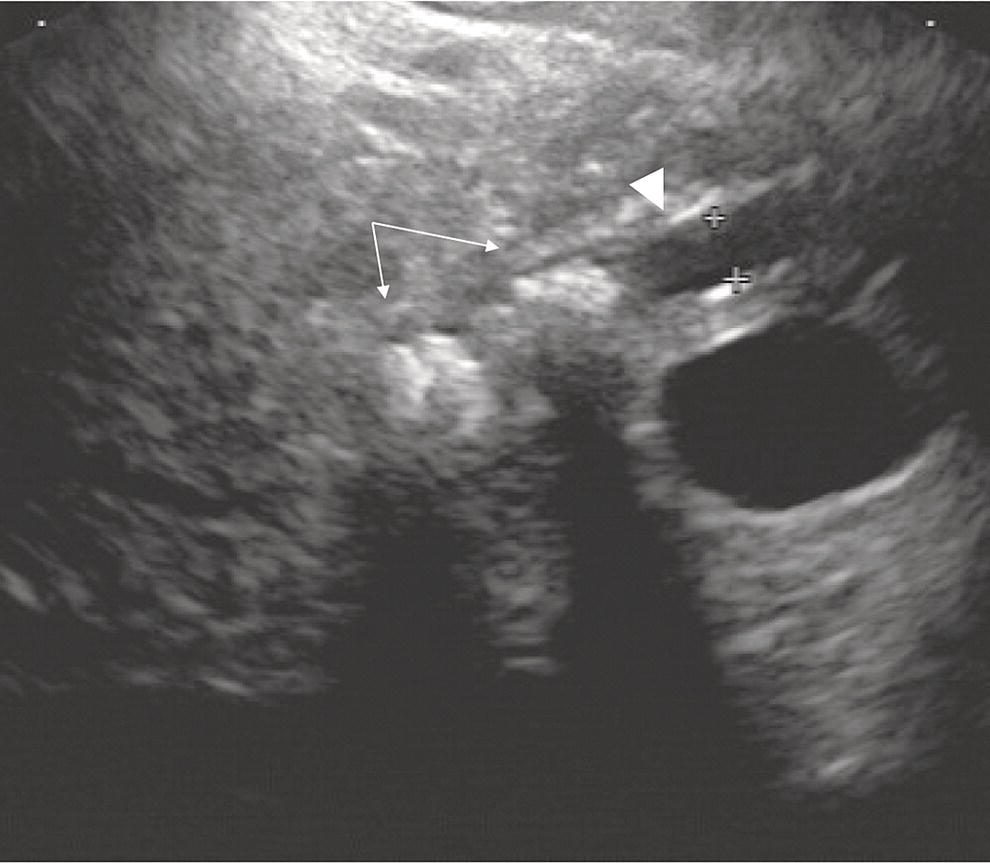
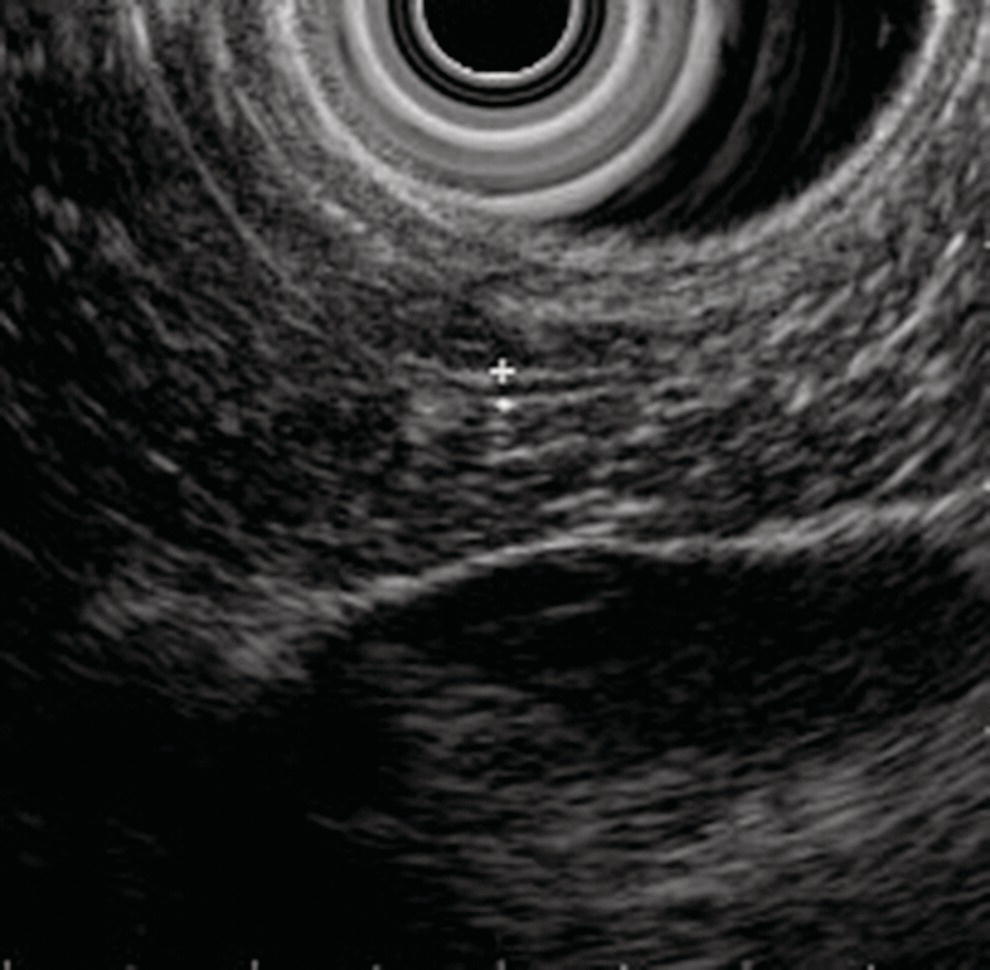
David G. Forcione Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA Chronic pancreatitis is defined by irreversible morphologic changes of the pancreas often associated with abdominal pain and, in late stages, endocrine and exocrine insufficiency. Data from the United States and Japan have demonstrated that as many as 3% of US male veterans and 17% of Japanese individuals may be affected. In addition to being associated with significant medical morbidity, care of patients with chronic pancreatitis is costly, with estimates of over $2 billion per year in the United States for the over 100 000 outpatients and 50 000 inpatient annual visits. As with other diseases, early diagnosis offers a better chance of effective treatment outcomes. Since Sivak’s original description of the use of endoscopic ultrasound (EUS) to evaluate chronic pancreatitis in 1986, there has been increasing interest and data demonstrating the utility of this imaging modality for early diagnosis. The close proximity of the upper gastrointestinal tract to the pancreas provides endosonographers with an ideal imaging modality of the pancreas. In general, EUS appears safer than endoscopic retrograde cholangiopancreatography (ERCP). Furthermore, there are increasing data that both ductal and parenchymal changes of the pancreas associated with chronic pancreatitis may be seen earlier and more accurately with EUS as compared with conventional cross‐sectional imaging modalities. Advances in EUS imaging such as sonoelastography and contrast‐enhanced EUS are expected to improve the overall accuracy of the examination. Preliminary data using combined contrast‐enhanced EUS and real‐time sonoelastography demonstrate these modalities may further improve our ability to discern chronic pancreatitis from pancreatic neoplasia. Threshold diagnostic criteria and interobserver variability are both common issues that may arise when any imaging modality is the predominant diagnostic tool for a specific disease entity. Chronic pancreatitis is particularly challenging given that the gold standard of diagnosis (i.e. histology) is rarely feasible. Furthermore, EUS is particularly susceptible as an imaging modality to these given the potential subjective nature of the examination. In the past two years, the EUS community has come closer to reducing the potential for this variability through the development of specific EUS‐based criteria. The Rosemont Criteria (2009) is a significant step forward towards unifying the role of EUS in diagnosing chronic pancreatitis. In this chapter, we review the utility of EUS as an imaging modality for chronic pancreatitis. The classification and diagnosis of chronic pancreatitis has evolved since the Marseilles conference of 1963 first established the histopathologic paradigm of fibrosis, inflammation, loss of exocrine parenchyma, ductal dilation, and stones. It soon became clear, however, that conventional radiologic diagnosis, i.e. transabdominal ultrasound (TUS), computed tomography (CT), and magnetic resonance imaging (MRI), was best suited for diagnosing advanced disease and may not have sufficient accuracy for early or mild chronic pancreatitis. The role of ERCP was incorporated in 1984 by the Cambridge Classification. The Cambridge Classification incorporates features of both the main pancreatic duct and side branch, including dilation and ductal ectasia, to assess the severity of chronic pancreatitis. Although ERCP appeared to be more accurate than conventional cross‐sectional imaging at identifying chronic pancreatitis through ductal changes, it continues to be seen as an imaging modality with limited predictive value. As noted with histopathology, autopsy series have demonstrated age‐related ductal changes of chronic pancreatitis without associated symptoms. Furthermore, as an invasive examination with potential morbidity, the role of ERCP for the diagnosis of early or mild chronic pancreatitis remains to be well established. Pancreatic function testing (PFT) remains an option for the diagnosis of chronic pancreatitis. Currently, this is performed using standard upper endoscopy and secretin stimulation of the exocrine pancreas. Duodenal aspirates are obtained for bicarbonate and enzymatic (lipase) measurements. Some have incorporated PFT in the diagnostic criteria for chronic pancreatitis. Nonetheless, the role of PFT remains uncertain due to limited availability, patient intolerance, and uncertain validation across larger, more diverse patient populations. Furthermore, patients may have exocrine changes without associated morphologic changes and it is unclear if these patient subsets truly have chronic pancreatitis. The gold standard for the diagnosis of chronic pancreatitis remains histopathology. Typical morphologic changes include chronic inflammation, fibrosis, and glandular atrophy. These findings may be uniform or patchy in the pancreas. Autopsy series have identified “asymptomatic/normal” individuals with significant atrophy and fibrosis. Furthermore, variation in pathologist interpretation of these findings adds to the potential difficulties in accurate diagnosis using these criteria. There is increasing evidence that a wide array of insults may be associated with the development of chronic pancreatitis. Whitcomb et al. established the TIGAR‐O classification in 2001 to incorporate the roles of Toxic (ethanol), Idiopathic, Genetic (SPINK1, PRSS, and CFTR), Autoimmune, Recurrent severe acute pancreatitis, and Obstructive etiologies. All of these entities should be carefully considered in patients being evaluated for chronic pancreatitis. Despite an exhaustive search, the etiology remains idiopathic in up to 20% of patients. In order to best understand the role of EUS in imaging of chronic pancreatitis, it is useful to review some of the fundamentals of normal pancreas imaging with EUS. Data from large series of “normal” TUS and EUS examinations from the 1990s have provided some useful imaging parameters. Wiersema et al. characterized the normal pancreas parenchyma as having uniform echogenicity, typically brighter than the liver. The mean ductal diameters were 2.4 mm in the head (range 0.8–3.6 mm), 1.8 mm in the body (0.9–3.0 mm), and 1.2 mm in the tail (0.5–2.0 mm). Catalano et al. made endosonographic observations of the pancreas in 25 “normal” patients. The normal pancreas parenchyma was described to be homogeneous with identification of variable echotexture separating the ventral and dorsal anlage of the pancreas in 68%. The mean ductal diameter in the body was 1.7 mm (range 1–3 mm), and side branches could be appreciated in 32%. Some studies have also provided some interesting insight into the wide spectrum of findings that may be seen in otherwise healthy individuals. A large TUS series from Japan (130 951 patients) identified that pancreatic duct dilation (>3 mm) may be seen in 0.49% of individuals and tended to be found in older men. In addition, calcifications and cysts were found in 0.05% and 0.22% of patients, respectively. In summary, these data provide endosonographers with a basic framework for normal parenchymal and ductal imaging and set the basis for establishing some threshold criteria for the diagnosis of chronic pancreatitis. Since the original description of the role of EUS imaging in chronic pancreatitis by Sivak in 1986, a number of studies have tried to define appropriate criteria for diagnosis. Early classifications incorporated nine criteria for chronic pancreatitis, including five parenchymal and four ductal. These data were almost exclusively derived using radial EUS imaging performed by experts in academic practices. The parenchymal changes included hyperechogenic foci, hyperechogenic strands, lobulation, cysts, and calcifications. Histopathologic correlates of the former three were felt to represent focal fibrosis and glandular atrophy. Ductal changes included a dilated main pancreas duct (>3 mm head, >2 mm body, >1 mm tail), ductal irregularity (“chain of lakes” appearance), hyperechoic ductal margins, and side‐branch ectasia (Figures 27.1 and 27.2). Figure 27.1 Endoscopic ultrasound (linear) showing features of chronic pancreatitis: a dilated pancreatic duct (arrowhead) and two intraductal stones (arrows). Figure 27.2 Endoscopic ultrasound showing a hyperechoic duct wall (crossmark) in chronic pancreatitis. Clearly, the number of threshold criteria selected will determine how sensitive and specific the imaging test may be: fewer (<2) threshold criteria are associated with higher sensitivity but lower specificity, and more (<6) are associated with a lower sensitivity and higher specificity. Since these criteria were developed, several studies have been done to define the appropriate threshold criteria. Initial attempts at this defined chronic pancreatitis to be “unlikely” if zero of nine criteria were noted, “likely” if more than five of nine were noted, and “uncertain” if between one and five criteria were noted. Both comparative and receiver operator curve studies have been done to further define the role of EUS and the number of threshold criteria. Stevens et al. compared EUS and ERCP with a threshold of six criteria and found statistical equivalence with ERCP in terms of sensitivity and specificity (68% and 79%, respectively). The authors also considered secretin stimulation pancreatic function testing (SS‐PFT). This did not appear to add to the diagnostic accuracy over ERCP and EUS. Furthermore, SS‐PFT was felt to be not widely available and potentially inconvenient for patients. Varadarjulu et al. evaluated patients with non‐calcific chronic pancreatitis going to surgery and identified more than four EUS criteria as 91% sensitive and 86% specific for the diagnosis. Pungapong et al. identified the combination of EUS and secretin‐stimulated magnetic resonance cholangiopancreatography (MRCP) to have a very high sensitivity and specificity at 98% and 100%, respectively. These authors also identified pancreatic juice interleukin‐8 levels (after secretin stimulation) to add diagnostic value. Finally, over the last five years, it also has become clear that age and other factors (gender, alcohol use, tobacco, body mass index) may affect the diagnostic threshold and that certain criteria, such as calcifications, may carry greater weight towards a diagnostic end point. As noted, histopathology remains the diagnostic gold standard for chronic pancreatitis. Unfortunately, this is rarely available outside of surgical or autopsy specimens. Biopsy techniques of the pancreas are limited by the often patchy nature of chronic pancreatitis, safety, availability, and limited tissue acquisition. Linear echoendoscopy provides an opportunity to perform biopsy (fine needle aspiration [FNA] for cytology and core biopsy for histology) of the pancreas using a transgastric or transduodenal technique, and this option has been evaluated for chronic pancreatitis. Hollerbach et al. evaluated EUS‐FNA in 27 patients with suspected chronic pancreatitis and found a surprisingly high specificity of 67%. Dewitt et al. evaluated the use of EUS Tru‐cut biopsy for core specimen acquisition in 16 patients with chronic pancreatitis. In this study, diagnostic confirmation was achieved in 5.6%. Fourteen of sixteen were either normal or non‐diagnostic and one was associated with equipment malfunction. The authors noted a complication frequency of 11%, including acute pancreatitis and pain requiring hospitalization in two patients. The overall kappa value in this study was only 0.25. At this time, the role of EUS‐FNA or Tru‐cut biopsy remains uncertain and limited by accuracy and technical factors (safety and availability). Over the last decade, the true role of EUS for the diagnosis of chronic pancreatitis has been hampered by a number of factors, including interobserver variability, lack of standardization in technique, and nomenclature. There has also been disagreement over the number and appropriate validation of threshold criteria. Furthermore, expense and availability of EUS has limited its impact for routine use in chronic pancreatitis. In 2007, a group of 37 expert endosonographers from North America and Japan convened in Rosemont, Illinois to review the literature and develop updated diagnostic criteria using EUS for chronic pancreatitis. The group evaluated three areas: parenchymal criteria, ductal criteria, and correlation with histology, and, from these, established an EUS‐based diagnostic system. For the first time, attention was also placed on the relative weight of various factors (i.e. major and minor criterion, and rank). Consensus for criteria was established when greater than two‐thirds agreement was found. It is important to note that similar to previous classification systems, the criteria were based on radial EUS imaging. This classification system (denoted the “Rosemont Criteria”) was formally published in Gastrointestinal Endoscopy in 2009. Table 27.1 The Rosemont Criteria. Source: adapted from Catalano MF, Sahai A, Levy M, et al. EUS‐based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009;69:1251–1261. The Rosemont Criteria are demonstrated in Table 27.1. Many of the previously described criteria were again found to be important imaging characteristics in diagnosing chronic pancreatitis. Hyperechoic foci with shadowing, cysts, and intraductal calculi were felt to be relevant if found anywhere in the pancreas, whereas all other findings required demonstration in the body and/or tail. After an extensive review of the literature and consensus discussions, the overall kappa for findings in this group of experts was quite good (>0.7). Some of the advantages of the Rosemont Criteria include differential weighting, stricter definitions, and a four‐level diagnostic stratification (Table 27.2). Since the publication of the Rosemont Criteria in 2009, one multicenter comparison study has been reported evaluating standard criteria versus Rosemont Criteria for chronic pancreatitis (Stevens et al.). In this study, 50 video clips (all radial EUS) were evaluated by 14 experts, equally divided into those with extensive experience (>1000 cases) and those with moderate experience (500–999 cases). In this series of clips, 13 had established chronic pancreatitis. The standard criteria threshold was more than four of nine findings. Interestingly, the use of the Rosemont Criteria did not significantly increase interobserver agreement for EUS diagnosis of chronic pancreatitis compared with standard scoring. One would therefore conclude that high interobserver agreement is critical for the reliability of any diagnostic test. Table 27.2 The Rosemont diagnostic stratification. Source: adapted from Catalano MF, Sahai A, Levy M, et al. EUS‐based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009;69:1251–1261. a Except non‐shadowing foci, cysts, main pancreas duct dilation, and dilated side branches. The Rosemont Criteria have initiated important steps in the right direction for the EUS diagnosis of chronic pancreatitis – a common nomenclature. Ideally, the system will be further validated in larger patient populations prospectively and against the gold standard of histopathology. The utility of EUS as a diagnostic modality for chronic pancreatitis holds great promise. Improvements in imaging quality will continue to evolve. Additional studies are clearly necessary to validate the Rosemont Criteria. Minimally invasive ways of pancreatic tissue acquisition will likely develop and will be an important part of the evaluation of these patients. As with many other complex diseases where tissue acquisition is challenging, multimodal evaluation may be optimal. It is likely that combination evaluation with high‐resolution EUS imaging, secretin‐stimulated MRCP, and exocrine function testing will provide the most accurate means of morphologic and physiologic assessment.
27
Chronic Pancreatitis
Introduction
Clinical overview of chronic pancreatitis
Endoscopic ultrasound imaging of the normal pancreas
EUS imaging in chronic pancreatitis: historical perspectives
EUS imaging in chronic pancreatitis: the Rosemont Criteria
Features
Definition
Location
Major criteria Minor criteria
Rank
Histologic correlate
Parenchymal
Hyperechoic foci with shadowing
Echogenic structures >2 mm in length and width that shadow
Body, tail
Major A
1
Parenchymal calcifications
Lobularity
Well circumscribed, >5 mm structures with enhancing rim and relatively echo‐poor center
2
Unknown
A. With honeycombing
Contiguous >3 lobules
Body, tail
Major B
B. Without honeycombing
Non‐contiguous lobules
Body, tail
Yes
Hyperechoic foci wthout shadowing
Echogenic structures/foci >2 mm in both length and width without shadowing
Body, tail
Yes
3
Unknown
Cysts
Anechoic round/elliptical structures without septations
Head, body, tail
Yes
4
Pseudocysts
Stranding
Hyperechoic lines >3 mm in length in at least two different directions
Body, tail
Yes
5
Unknown
Ductal
MPD calculi
With acoustic shadowing
Head, body, tail
Major A
1
Stones
Irregular MPD
Uneven or irregular MPD
Body, tail
Yes
2
Unknown
Dilated side branches
>3 tubular anechoic structures each >1 mm width budding from MPD
Body, tail
Yes
3
Side branch ectasia
MPD dilation
>3.5 mm body, or >1.5 mm tail
Body, tail
Yes
4
MPD dilation
Hyperechoic MPD margin
Echogenic, distinct structure >50% entire MPD
Body, tail
Yes
5
Ductal fibrosis
Stratum
Criteria
Consistent with chronic pancreatitis
1 major A + >3 minor features
1 major A feature + a major B feature
2 major A features
Suggestive of chronic pancreatitis
1 major A feature + <3 minor features
1 major B feature + >3 minor features
>5 minor features
Indeterminate of chronic pancreatitis
3 or 4 minor features, no major features
Major B alone or with <3 minor features
Normal
<3 minor featuresa, no major features
Endoscopic ultrasound imaging in chronic pancreatitis: the future
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree