Superior soft tissue and contrast resolution of MR imaging benefits sensitivity to kidney cyst features and classification, which may have an impact on patient management and outcomes. Contrast-enhanced ultrasound (CEUS) may have nearly similar sensitivity for detection of cyst features yet is dependent on patient body habitus and adequacy of visualization windows for the kidneys, which does not have the same impact on MR imaging results. Both MR imaging and CEUS may provide superior kidney cyst assessment compared with contrast-enhanced CT; however, further research is needed, particularly for the identification of role of CEUS.
Key points
- •
Superior soft tissue and contrast resolution of MR imaging benefits sensitivity to kidney cyst features and classification, which may have an impact on patient management and outcomes.
- •
Contrast-enhanced ultrasound evaluation is a safe alternative imaging modality for the assessment of complex cystic renal masses in patients with renal impairment.
- •
Further research is needed to identify the role of contrast-enhanced ultrasound in the differentiation of benign complex cystic lesions from malignant complex cystic lesions and to determine its accuracy compared with MR imaging.
- •
Determination of subtypes of cystic renal cell carcinomas by imaging is still challenging due to overlap of common imaging features.
Introduction
Renal cysts are common incidental findings on imaging studies performed for nonurologic indications. Autopsy series report 50% incidence of at least 1 renal cyst in adults. Commonly encountered cysts on ultrasound (US) and cross-sectional imaging (CT and MR imaging) mostly are benign simple cortical renal cysts. Complex cysts, on imaging, may be seen as part of benign cysts, renal infection, and cystic neoplasms, including cystic renal cell carcinoma (RCC), which can be distinctly identified due to characteristic imaging features.
Cystic renal tumors, overall, have excellent prognosis and less aggressive metastatic behavior compared with solid renal tumors. Imaging plays a critical role in management, differentiating benign cysts, which do not require treatment, from cystic neoplasms, which may benefit from partial or radical nephrectomy, or percutaneous ablation under imaging guidance. Neoplastic cysts show complex features that include wall thickening, intracystic lobulated soft tissue, irregular thick cyst walls with thickened septations, and with these soft tissue components demonstrating enhancing elements on contrast-enhanced imaging.
Bosniak classification of renal cysts
Since its introduction in 1986, Bosniak classification for renal cysts has been used by radiologists and urologists as a method to stratify patients to plan treatment. Bosniak classification is based on findings on contrast-enhanced CT scans, dividing renal cysts into 4 broad categories (I–IV) proposed to distinguish malignant from benign cystic lesions. In 2005, a new II-F category was added to classification to the Bosniak classification system.
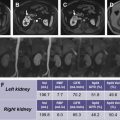
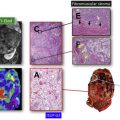
Bosniak category I represent cysts are simple and outlined by a thin wall, without internal septa or contrast enhancement ( Fig. 1 ). Category II corresponds to cysts with thin septations, minimally smooth thick walls but without contrast enhancement or nodularity ( Fig. 2 ). Category III includes complex cysts with irregular and/or thick septa, coarse calcifications, and presence of enhancement after injection of iodinated contrast ( Fig. 3 ). Enhancing well-defined solid components and thickened septations with irregular cyst walls comprise category IV ( Fig. 4 ). Category II-F is assigned to indeterminate lesions, between category II and III, which are clearly not benign, and warrant follow-up imaging (“F” refers to follow-up). These patients require several follow-up examinations for longitudinal evaluation, allowing the natural course of growth to reveal a neoplasm warranting intervention. These multiple CT studies expose patients to a cumulative x-ray dosing and risks related to iodinated contrast. The Bosniak classification system has been extended to apply to contrast-enhanced ultrasound (CEUS) and gadolinium contrast-enhanced MR imaging.




MR imaging has excellent intrinsic soft tissue and contrast resolution, which makes the appearance of septa more conspicuous and enhancement of the solid components, septa, and cystic walls more evident. This may yield higher Bosniak classification grading compared with CT. Graumann and colleagues report MR imaging both upgraded and downgraded 22% of the cases in their series, because of better contrast resolution, which aided distinction of solid and cystic elements within cysts. Higher-contrast resolution of CEUS, coupled with microbubble postcontrast imaging, helps identify details within a cyst (solid nodules, septa, cystic walls, and enhancement of soft tissue). Compared with the same CT study cohort, CEUS also both upgraded and downgraded 21% of the lesions, suggesting superior results to CT and similar results to MR imaging, when comparing delineation of solid and cystic components.
Contrast-enhanced ultrasound
US is easily available and frequently used for imaging kidneys. Complex cysts may show internal septations, solid components, or thickened walls, with vascularity of these soft tissue elements assessable with duplex Doppler technique. US contrast agents have been recently used in assessing blood flow with solid renal masses and soft tissue components of complex cysts masses. Vascularity in fine septations and small mural nodules may be challenging to elicit on conventional US using duplex Doppler. Enhancement can be improved, however, after contrast administration with use of tissue harmonic imaging in real time, differentiating benign complicated cysts from cystic tumors.
US contrast comprises synthetic microbubbles with diameters in the range of 1 μm to 10 μm, up to the size of red blood cells. These microbubbles are small enough to flow through capillaries but too large extravasate into the interstitium. The distribution volume represents the intravascular compartment. Thus, microbubbles are useful for determining vascularity, perfusion, and angiogenesis. Microbubbles are typically composed of a phospholipid shell containing low solubility complex gas, such as a perfluoro gas. Conventional B-mode sonographic frequencies can burst the microbubbles. Therefore, it is essential to use low mechanical index US waves to capture postcontrast sonographic imaging. Both linear and nonlinear oscillations occur in the microbubbles after exposure to low mechanical index US, which leads to generation of harmonics overtone signals from the blood due to increased backscatter. Generation of signal from microbubbles is independent of blood flow or vessel diameter and is based on US imaging with optimized spatial resolution. These characteristics of CEUS differentiate from duplex Doppler US for the evaluation of smaller vascularized soft tissue structures, which may not be resolved by Doppler due to lower intrinsic spatial resolution.
Microbubbles have a short half-life (approximately 5 min) after intravenous injection. Usual doses are 1 mL to 2.4 mL of microbubble preparation, suspended in saline, followed by a saline bolus. The microbubbles are disintegrated into its constituting parts; phospholipid shells are metabolized by the liver and the gas exhaled by the lungs.
CEUS gives an opportunity to image tissue vascularity (including arterial) in real-time, rapid, and continuous fashion over several minutes. More than 1 injection may be administered safely to evaluate both kidneys. US contrast agents have the benefit of being safe and with less severe adverse effects, compared with CT or MR contrast agents. Microbubbles are not hepatotoxic or nephrotoxic and can be given to patients with renal insufficiency and has a very good safety profile in terms of adverse reactions.
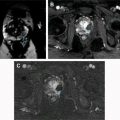
CEUS can reveal details of cyst wall, including nodularity and thickness, accurate number and thickness of septations, and the presence of enhancing solid components ( Fig. 5 ). Wash-in times and peak-to-enhancement values can used to assess enhancement patterns. An increasing body of literature supports that CEUS is as good or better in showing enhancing fine septa in cystic renal masses than CECT. Xu and colleagues were able to differentially characterize benign and malignant cystic renal masses based on CEUS appearances. The presence of enhancing nodules was the most common feature leading to Bosniak category IV classification and subsequent correlation with histologic subtypes of malignant cystic tumors (cystic clear cell RCC [cystic CC-RCC] and cystic papillary RCC [cystic P-RCC]). A multilocular cystic nephroma (MLCN) was classified as Bosniak category III, based on the multiloculated appearance and the lack of solid components in this particular study.

Magnetic resonance imaging
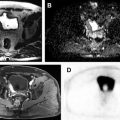
MR imaging in recent years has established a valuable role in the evaluation of cystic renal lesions due to its superior contrast resolution. Fluid-sensitive T2-weighted (T2W) sequences provide exquisite details of contents within renal cysts, including nodularity, septation, and solid components. Precontrast and dynamic postcontrast fat-saturated (FS) T1-weighted (T1W) 3-D gradient-echo (GRE) sequences are valuable in identifying enhancement of septation and/or solid nodules, which indicate malignancy.
Diffusion-weighted imaging (DWI) relies on the random motion of free water molecules and it has been widely used for the characterization of benign versus malignant processes in the abdomen. DWI and apparent diffusion coefficient are now increasingly used for the evaluation of kidney tumors, including categorization and staging of solid renal tumors. Solid components of cystic tumors show restriction on DWI, aiding accurate identification of malignant cystic tumors.
Rosenkrantz and colleagues report increased demonstration of septations, septal and/or mural thickening and irregularity, and mural nodules in cystic renal tumors using 3T MR imaging, compared with 1.5T and suggested more accurate cyst classification on 3T MR imaging. If 1.5T MR imaging is used, these investigators have recommended longitudinal evaluation of a patient should be performed at a consistent field strength, thereby leading to consistent evaluation.
Cystic renal tumors
Imaging plays a crucial role in stratifying renal cystic lesions, into benign simple cysts, mildly complex benign cysts (blood-protein cysts), or complicated cysts representing low malignant-potential tumors (MLCN or multilocular cystic RCC) and malignant cystic renal tumors (CC-RCC and P-RCC) with internal necrosis, cystic change, or focal hemorrhage. Identification of lesion complexity has a critical impact on patient management. Although nephron-sparing surgery is offered to low malignant-potential tumors (MLCN or multilocular cystic RCC), nephrectomy/radical nephrectomy may be considered in larger malignant tumors.
Renal Cell Carcinoma
A rising incidence of RCC has been observed in recent years, which may be attributed to both increasing exposure to risk factors and increasing use of imaging technologies. RCC represents a spectrum of disease, encompassing a family of differing neoplasms with different cytogenetic and molecular defects and varying prognoses and potential morbidities. The World Health Organization (WHO) classification divides these tumors into different classes based on histologic, immunohistochemical, and genetic differences as follows :
- 1.
CC-RCCs (65%)
- 2.
P-RCCs (15%; 90% 5-year survival in sporadic cases)
- 3.
Chromophobe RCCs (5% overall best prognosis)
- 4.
Collecting duct carcinomas (1%; very poor prognosis)
- 5.
Renal oncocytomas (5%; benign tumors, similar pathology to chromophobe tumors except by immunohistochemistry)
- 6.
Unclassified tumors (5%)
Cystic clear cell carcinoma
CC-RCC is the most common subtype of RCC. A majority of CC-RCC are solid masses; only approximately 15% may have cystic change. Hartman and colleagues characterize 4 basic pathologic mechanisms resulting in cystic RCC: intrinsic multiloculated growth, intrinsic unilocular growth (cystadenocarcinoma), cystic necrosis (pseudocyst), and origin from the epithelial lining of a preexisting simple cyst. Based on these histologic appearances, CC-RCCs can be classified into 4 morphologic subtypes, seen on imaging:
- 1.
Multilocular cystic RCC
- 2.
Unilocular cystic RCC
- 3.
Cystic RCC with 1 or more mural tumor nodules
- 4.
RCC with extensive necrosis
Multilocular cystic renal cell carcinoma, also known as multilocular cystic renal neoplasm of low malignant potential
Multilocular cystic RCC is a rare subtype, first described in 1982, and comprising up to 6% of RCCs and 15% to 40% of all CC-RCCs. These asymptomatic tumors are incidentally discovered in adults (20–76 years of age) with a favorable prognosis, without local recurrence or metastatic disease after resection.
Multilocular cystic RCC is a low-grade tumor comprising cysts of varying sizes that are separated from the renal cortex by a fibrous capsule, which contains serous or hemorrhagic cyst fluid. The cyst wall is lined by a monolayer of epithelial cells with clear cytoplasm.
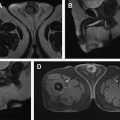
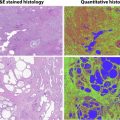
On MR imaging, these tumors show a T2-hyperintense, well-defined, multilocular cystic structures. FS T1W 3-D GRE postcontrast images reveal enhancement of the cyst wall and septa, without any internal mural enhancing nodules ( Fig. 6 ). Aubert and colleagues, in their investigation of preoperatively diagnosed cystic clear cell carcinomas, were able to distinguish cystic CC-RCC from multilocular cystic RCC by the presence of fewer loculations, thicker and more nodular septa, and enhancing mural nodules. Hindman and colleagues, however, report overlapping imaging appearances of multilocular cystic RCC to cystic neoplasms, including cystic nephroma, mixed epithelial and stromal tumor (MEST), extensively cystic RCC, tubulocystic carcinoma, and P-RCC. Most of the peer-reviewed literature lacks validation of MR imaging appearance of cysts by correlative histopathology.