6
Clinical and Noninvasive Evaluation of Peripheral Vascular Disease
Evolution in technology surrounding vascular disease has resulted in increased treatment options for persons afflicted with peripheral vascular disease (PVD). Decreased morbidity and mortality associated with newer procedures have led to broadening of the indications for the treatment of these patients. Additionally, recent innovations in vascular imaging have enhanced the ability to detect vascular disease using less invasive techniques. Despite these advances, optimal care of the patient with PVD still requires a balanced approach between conservative therapy and more invasive procedures. Maintaining this balance necessitates an ongoing process of evaluation of the disease process and its effects on the patient. The importance of the clinical evaluation cannot be overstated. In many cases, the clinical presentation may be straightforward and a tentative diagnosis made on the basis of a directed history and physical examination. In others, however, the diagnosis may be less certain. Objective data supplied by the noninvasive vascular laboratory can be of great use in evaluating patients with suspected PVD, not only documenting the presence of disease but also providing information about the location, severity, and etiology of the disease process. Conversely, data supplied by the noninvasive vascular laboratory should be viewed in the context of the clinical presentation. Only then can appropriate decisions regarding therapeutic options be made.
Evaluation of the patient with PVD requires an understanding of the pathophysiology and natural history of the disease. PVD is a disease of the aging population. Its frequency increases rapidly with age, from 3 to 5% in patients under 60 years of age to more than 20% in patients over 75 years of age.1–5 Many patients decrease their activity level with advancing age; therefore most cases of PVD in the general population are not symptomatic and pose no threat to the patient. An estimated four of five patients with demonstrable PVD are asymptomatic.3–4 Other patients with PVD present with varying clinical manifestations, intermittent claudication being the most frequent presenting symptom. It is important to realize that PVD generally runs a benign course: 75% of patients will stabilize (60%) or improve (15%) their clinical status without intervention following their initial presentation.6 Fewer than 25% will have significant clinical progression. Amputation is infrequent: Only 5 to 6% of patients progress to this point over a 10-year period.6–7 Patients who smoke cigarettes and who have diabetes have a greater risk. Long-term follow-up of these patients reveals an amputation rate of 20% or greater.1,8 With regular exercise and risk-factor control, however, most patients will demonstrate significant improvement.9,12 A study of patients undergoing a supervised exercise program established not only clinical improvement but also evidence of significant metabolic improvement after 12 weeks.9
The risk factors for PVD are well known: hypertension, smoking, diabetes mellitus, abnormal cholesterol levels, obesity, and a strong family history.1,3–5 Men are at higher risk than women, although this difference is reduced with advancing age. The presence of concomitant vascular disease in other organ systems cannot be ignored. Increased morbidity and mortality among patients with intermittent claudication are well documented.1–3,5–6,8,13–17 One third of patients with claudication die within 10 years of their presenting symptoms, and two thirds of these patients will have experienced a major cardiovascular event. A review of patients undergoing treatment for PVD reveals a mortality rate even higher than the general population with claudication: 5-year mortality rates are 25 to 40% and 10-year rates, 50 to 75%.1,3,5,8,13 The vast majority of these deaths are secondary to atherosclerotic heart disease. In one study of patients who underwent vascular surgery, all 14 patients with diabetes mellitus and coronary artery disease died within 5 years.18 Similarly, in a second study, all patients with diabetes mellitus who had undergone aortofemoral reconstruction died within 5 years of surgery.19 Clearly, although intermittent claudication itself is generally benign, the associated ramifications are markedly increased morbidity and mortality compared with the general population, in large part as a result of the effects of atherosclerotic disease on other organ systems, mainly the coronary and cerebral vasculature. Risk-factor modification should be a significant part, if not the mainstay, of any therapeutic regimen. Attempts to modify risk factors should be initiated, beginning with patient evaluation.
 Clinical Evaluation
Clinical Evaluation
Evaluation of the patient with suspected PVD should begin with a directed history and physical examination. Much can be determined simply by listening to the patient’s description symtoms. Relevant details such as onset (gradual versus acute), character, location, and aggravating and relieving factors should be sought. Combining the clinical history with findings extracted during physical examination will aid in determining the nature and extent of the disease process.
History
Intermittent claudication, reported by about 70% of patients at initial presentation, is the most frequent complaint of patients with PVD.2–5 Claudication (from the Latin word claudicatio, meaning to limp) is defined as muscular pain brought on by exercise and relieved by rest. Onset is predictable in terms of the activity level required to produce the symptoms. Similarly, the symptoms rapidly resolve with rest of the extremity. There is generally no relation to position of the extremity. The discomfort itself tends to be described by patients as a “cramping” pain in the body of the muscle; however, the perception may vary from patient to patient. The quality of discomfort may range from a sharp searing or stabbing pain to an aching discomfort. Others complain of numbness, heaviness, fatigue, or weakness. Vasculogenic claudication must be differentiated from other states, such as neurogenic claudication and arthritis.20 Neurogenic claudication or pseudoclaudication may be associated with spinal stenosis. Therefore, there is generally less predictability regarding onset with exercise. Interestingly, although these patients may be limited in their ability to ambulate, they may experience no limitation with alternative forms of exercise, such as an exercise bicycle, because of the postural changes associated with the alternative exercises. Additionally, in contradistinction to vascular claudication, relief frequently is delayed following cessation of exercise and may require additional change in position.20 Unfortunately, because both of these diseases are increasingly common with advancing age, they may coexist. Similarly, arthritic complaints, usually joint centered, may be confused with claudication. Onset may occur after rather than during exercise, and relief is delayed and often positional. The noninvasive vascular laboratory with exercise testing can be extremely useful in differentiating true vascular claudication from other conditions.
Claudication characteristically develops in the muscles of the calf, although other sites may be involved. In part this relates to the heavy dependence on the calf muscles during walking, frequently the only source of significant exercise in the aging population. Distribution of disease also plays a significant role. Although younger patients affected by PVD frequently present with an aortoiliac distribution, the femoropopliteal system more frequently is involved in the older population.4,21 In fact, multisegment disease is common in patients who have PVD symptoms. Because the effects of disease on perfusion are hemodynamically cumulative as one progresses distally in the arterial tree, it is reasonable that the calf muscles are frequently the site of most significant ischemia when walking. Other sites of claudication occasionally dominate. Young women frequently present with lesions involving the distal aorta and its bifurcation.22 With proximal disease, patients may complain of symptoms involving the buttocks or thighs.21 Similarly, men with disease in this distribution may experience erectile dysfunction. Conversely, foot claudication may be the presenting symptom in patients with isolated distal vessel disease, such as Buerger’s disease.23,24 Unusual character or distribution of symptoms may be the cause of significant delay in diagnosis and treatment.21
As the degree of ischemia worsens, the limitations imposed on the patient increase, and symptoms may occur at rest. Ischemic rest pain occurs when perfusion of the extremity is inadequate to meet the basic metabolic needs of the tissues.25 Because perfusion status is extremely tenous at this point, small changes in perfusion brought about by positional changes may either alleviate or aggrevate symptoms. The patient may describe the need to hang the distal extremity over the edge of the bed at night for symptomatic relief provided by the gravitational advantage in perfusion. Frequently, the patient may need to get up at night to “walk off” the pain, seemingly a contradiction. Minor trauma to the extremity with this level of impaired perfusion may result in tissue break-down or cutaneous ulceration. A small cut or blister that is generally an innocuous event in the otherwise healthy patient may become a limb-threatening lesion. Similarly, with significant ischemia, cellulitis can progress rapidly despite adequate antimicrobial treatment. Revascularization of these extremities is critical to long-term limb salvage and, depending on the clinical situation, should not be delayed. Further decrease in perfusion invariably will lead to tissue loss. Although tissue damage at this point may be irreversible, limb salvage still can be attained with revascularization and wound care.
Symptoms of chronic limb ischemia generally have an insidious onset, although patients frequently relate the onset to some event in their lives. As might be expected, patients presenting with rest pain usually relate a long history of worsening claudication before, seeking medical help. Ischemia, however, may present in an acute fashion, often with abrupt onset of severe pain in the calf or foot. Depending on the severity of the ischemia, the patient may complain of paresthesias or numbness rather than pain or describe sudden onset of coolness and discoloration with either cyanosis or pallor. Motor function also may be impaired; the patient may be unable to move the foot or toes. Generally, the symptoms are so sudden and severe that medical attention is sought immediately. If there is delay in presentation, however, the acute, severe symptoms may subside to some degree because collateral beds gradually supply some reperfusion to the affected limb. Alternatively, without improved perfusion, clinical status may deteriorate rapidly, with tissue breakdown and the development of gangrenous changes.
Acute ischemia may be secondary to an embolic or thrombotic event. Emboli can occur anywhere in the arterial tree but frequently lodge at sites of arterial division where vessel caliber is reduced suddenly. Large emboli may adhere to the aortic bifurcation, affecting both lower extremities. The common femoral bifurcation and the popliteal trifurcation are two additional frequent sites of emboli, each presenting with a different distribution of ischemia. Occasionally, microembolization will occur to the most distal vascular beds. The sudden appearance of one or more painful, discolored toes in the presence of palpable pedal pulses, called the “blue toe syndrome,” is characteristic of atheroembolization to the digital arteries (Fig. 6-1).26 In general, this condition is thought to represent embolization from a proximal ulcerated atherosclerotic lesion. Depending on the severity of the proximal lesion, there may be an antecedent history of claudication. Occasionally, a similar presentation occurs secondary to a proximal critical stenosis without demonstrable ulceration. Presumably, small thromboemboli account for the distal arterial occlusions. A thorough history may reveal prior episodes of distal embolization. Even in the presence of palpable pulses, distal embolization warrants further investigation of the source of em boli. Many of the inciting lesions are treatable by percutaneous techniques.
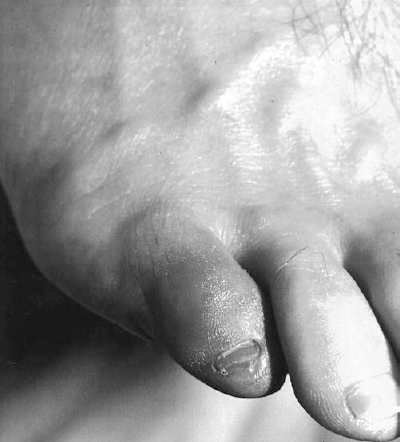
FIGURE 6-1. A 48-year-old man presented with the acute onset of a painful discolored fifth toe. A focally ischemic digit in the face of normal pulses is characteristic of the “blue toe syndrome.” Note the normal cutaneous features, hair distribution and venous distention indicating lack of chronic ischemia.
Thrombosis as a cause of acute ischemia may involve native vessels or bypass grafts. If there is thrombosis of a vessel with preexisting stenosis, careful questioning frequently will yield a preceding history of claudication. The differentiation of in situ thrombosis of a diseased vessel from embolic occlusion of an otherwise healthy vessel carries different prognostic and therapeutic implications. Thrombectomy is much less likely to be successful if the underlying vessel is diseased; thrombolysis and angioplasty may represent an attractive alternative. In addition to stenotic disease, aneurysmal disease is particularly susceptible to sudden thrombosis and or embolization.27,28 Acute ischemia secondary to popliteal artery thrombosis should lead to an investigation of the underlying artery (as well as the contralateral artery) to exclude aneurysmal disease. A similar picture may be encountered with popliteal entrapment with or without associated aneurysm formation. Again, examination of the contralateral extremity is crucial, because the disease is frequently bilateral. Bypass grafts frequently fail without prior clinical symptoms, and a history of claudication may not be encountered. Routine graft surveillance is therefore thought to be warranted to avoid the consequences of graft failure.29,30
Further interrogation regarding the involvement of vascular disease in other organ systems should be undertaken. As previously noted, cardiovascular and cerebrovascular events account for most of the mortality in this patient population. The presence of significant disease involving these organ systems may alter the therapeutic approach to the patient. Risk factors should be elucidated, and initial efforts at risk-factor modification begun. Family history is important not only for its prognostic value, but it also occasionally aids in determining the source of disease. A family history of thrombotic events may suggest a hypercoagulable state, although this is found infrequently. These events more often involve the venous system but occasionally result in arterial emboli or thrombosis, particularly following instrumentation, such as arterial catheterization or vascular surgery. Surgical history, particularly as it pertains to the vascular system, should be outlined in detail. Prior grafts may alter significantly both diagnostic and therapeutic approaches. Unusual graft anatomy or occlusive disease can interfere with angiographic and therapeutic approaches. Prior harvesting of the saphenous vein may limit surgical alternatives.
Current medications, particularly those with vascular effects, such as anticoagulants and vasoconstricting agents, should be recorded. Drug interactions and side effects are not infrequent and may have consequences for therapeutic endeavors. Specific inquiry must be made regarding certain drugs (e.g., nicotine patches) because often the patient does not perceive these agents as being medication
Physical examination
With the clinical history in mind, a directed physical examination should be performed to evaluate the patient’s vascular status. The investigation should include a basic cardiovascular examination as well as an evaluation of the possible effects of vascular disease on the extremities. Ausculatation of the heart is performed to exclude arrhythmias such as atrial fibrillation and significant murmurs. The carotid arteries, abdomen, and pelvis, including the femoral arteries, are auscultated for the presence of bruits. A thorough assessment of peripheral pulses by palpation includes both upper and lower extremities. Pulses should be recorded as absent, diminished, normal, or hyperdynamic bilaterally. If a pulse is absent by palpation, its presence should be ascertained by using a handheld Doppler device. Although the dorsalis pedis pulse may be absent by palpation in up to 12% of the normal population, absence of the posterior tibial pulse is a strong indicator of disease.4 The presence of significant soft tissue edema or open ulceration may confound the process of palpation; again, Doppler evaluation may be necessary. Palpation also should be used to investigate possible aneurysmal disease at common sites of formation including the abdominal aorta, common femoral arteries, and popliteal arteries (the most frequently ignored site). Blood pressures obtained from both upper extremities should be recorded as part of the vascular examination.
Inspection of the extremities is performed to evaluate for signs of acute and chronic arterial insufficiency. In addition to pulse evaluation, this should encompass inspection of the quality of the skin, the distribution of hair on the extremities, cutaneous temperature, capillary refill and neuromuscular function, each as it relates to the presence of vascular disease. Chronic arterial insufficiency leads to trophic skin changes with thin, shiny skin, particularly in the pretibial region.11 Loss of normal hair distribution occurs from the distal calf and dorsum of the foot. Nails may become thickened and brittle. With increasing severity of chronic ischemia, dependent rubor may be present owing to relatively fixed vasodilation in the distal arteriolar and capillary beds. With the patient in a sitting or erect position, the foot and distal calf are ruborous, whereas in the supine position, particularly with the extremity elevated, the extremity appears pale. Temperature changes, which are more pronounced in the acutely ischemic limb, may be present in chronic ischemia, particularly if the limb is left without the thermal protection of a sock or blanket for a brief period. Comparison of proximal to distal and side-to-side is made. Capillary refill is evaluated by applying gentle pressure to the skin or nail bed, followed by release and observation of the return of the normal pinkish color. Capillary refill, normally 1 to 2 seconds, becomes increasingly delayed with the severity of the ischemia. As ischemia progresses and the limb becomes threatened, sensory and muscular function are affected. These changes are generally most pronounced in acute, severe ischemia. With chronic ischemia, muscle mass begins to atrophy, accounting for much of the weakness encountered. Finally, inspection should include a thorough evaluation of skin integrity. Tissue breakdown, ulceration, and frank gangrene may occur with severe ischemia. Ulceration tends to occur at pressure points in the distal extremities, including the regions around the malleoli, heels, heads of the metatarsals, and toes. Frequently undetected without careful inspection are lesions between the toes. Detection of tissue breakdown is extremely important because these lesions, untreated, may become infected and life threatening. Ischemic ulcerations tend to be dry, punched-out lesions with well defined borders (Fig. 6-2). Usually little erythema is found unless infection is present. These ulcerations should be differentiated from venous ulcers, which tend to be weeping, indurated lesions around the distal calves (Fig. 6-3). Associated cutaneous changes of venous stasis are frequently present with brawny edema and brownish discoloration of the skin. Dilated superficial varicosities also may be in the region of a venous ulcer.
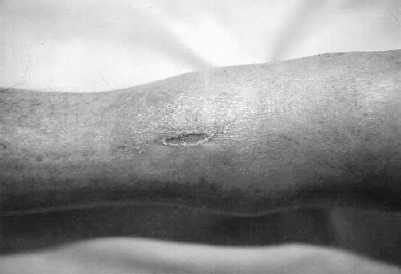
FIGURE 6-2. A 68-year-old woman presented with a chronic ulcerated lesion along the anterior aspect of her shin. The lesion is well defined, dry and shows little associated erythema. The surrounding skin is atrophic with a paucity of hair. Although unusual in position, the lesion is characteristic of an ischemic ulcer.
The physical examination in acute limb ischemia may be quite different from that of chronic disease. Initially, pulses are severely diminished to absent because collaterals have had little time to form. The underlying skin and hair distribution are frequently normal, although in the case of acute graft occlusion, the stigmata of chronic disease may have previously developed. Color change may be pronounced, with the distal ischemic portion of the extremity having a blanched or marbled appearance in the more severe cases. Differential temperature of the affected extremity is usually pronounced. The level of temperature change should be recorded as well as marked on the extremity. As the ischemic process evolves, a well-defined line of demarcation develops between the perfused proximal extremity and the ischemic distal portion. As collateral perfusion increases following the acute episode, this line of demarcation tends to move distally. Capillary refill may be markedly delayed or absent. Evaluation of motor and sensory function is critical in these patients because it aids in the determination of limb viability. Sensory changes range from none to complete anesthesia. Patients with threatened but reversible ischemia frequently note dysethesia or paresthesia of the affected extremity. The presence of diabetes may confuse the issue because peripheral neuropathy is frequently present.31 Comparison with the contralateral extremity should be performed in all patients but is particularly useful when an underlying neuropathy is present. Similarly, muscle function ranges from normal to paralysis; weakness is a sign of the threatened limb. Generally, tissue breakdown is not present at initial presentation owing to the acuity of the process. Without revascularization, however, the threatened to irreversible limb may progress rapidly to frank gangrenous changes. When present, these changes may represent a contraindication to revascularization, particularly if infection is present, owing to the milieu of toxic substances that may be released from the affected limb.
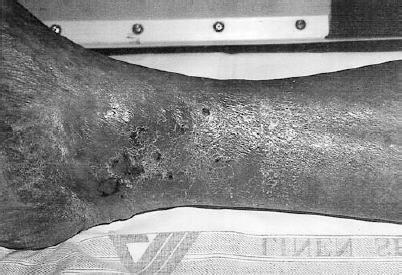
FIGURE 6-3. A 58-year-old black man presented with chronic cutaneous ulceration around the medial malleolus. The reddish brown discoloration of the skin and the distribution around the ankle are typical of chronic venous stasis changes.
To provide consistency in the evaluation and reporting of the ischemic limb, a system of grading extremities has been developed for both acute and chronic limb ischemia (Table 6-1) using clinical findings and objective criteria that may be provided by the vascular laboratory.32–35 In addition to prognostic implications, therapeutic decisions can be made consistently using these standardized categories. For instance, percutaneous thrombolytic treatment of the acutely threatened limb may be appropriate in many cases. If the clinical status of the extremity declines rapidly, with loss of sensory and motor function, there is frequently insufficient time for thrombolytic agents to restore perfusion, and surgical revascularization may be more appropriate. The goal of the clinical evaluation of the patient is to document the vascular status of the patient, providing a basis for any therapeutic decision, whether conservative or invasive.
Noninvasive Vascular Testing
Although the importance of the clinicalevaluation cannot be overstated, objective data supplied by the noninvasive vascular laboratory can be of great use in the evaluation of patients with suspected PVD, not only documenting the presence of disease but also providing information as to the location, severity, and etiology of the disease process. For instance, there may be a paucity of physical findings in many patients with intermittent claudication. The absence of peripheral pulses is an unreliable finding. Patients with high levels of activity may be severely limited despite the presence of palpable peripheral pulses on resting examination. Therefore, a diagnosis of intermittent claudication is sometimes difficult, with history and physical examination having false-positive and false-negative rates in the range of 44% and 19%, respectively.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree