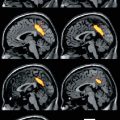
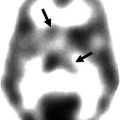
Fig. 33.1
[123I]FP-CIT SPECT scan obtained (3 h after injection) in a 21-year-old healthy female control (left side) and in a 78-year-old healthy female control (right side). Striatal binding of [123I]FP-CIT is symmetrical and clearly visible in both the caudate nucleus and putamen, in the young as well as the aged healthy control
Some [123I]FP-CIT SPECT studies reported higher striatal binding ratios in women than men (Lavalaye et al. 2000; Eusebio et al. 2012), although this was not observed in all [123I]FP-CIT studies. Also, an [123I]β-CIT SPECT study performed in a large cohort of healthy controls (70 males and 52 females) did not find a significant effect of gender on striatal binding ratios (Best et al. 2005). On the other hand, a large [123I]FP-CIT SPECT study did demonstrate higher striatal binding ratios in women than men (Varrone et al. 2013). Taken together, these data suggest that the diagnostic accuracy of [123I]FP-CIT SPECT in routine clinical studies may be improved by taking gender effects into account.
[123I]β-CIT SPECT studies in healthy controls demonstrated that the level of striatal DAT availability in the human brain may be associated with polymorphisms in the gene encoding for the DAT (van de Giessen et al. 2009; van Dyck et al. 2005; Jacobsen et al. 2000). However, a recent meta-analysis failed to establish a significant relationship between in vivo striatal DAT availability and polymorphisms in the gene encoding for the DAT in healthy controls (Costa et al. 2011). Also, to our knowledge no [123I]FP-CIT SPECT study has been performed to look into this topic in a large group of healthy controls. Consequently, as it stands now, there is insufficient support to take gene effects into account in a setting in which [123I]FP-CIT SPECT imaging is used for routine diagnostic purposes.
33.3 [123I]FP-CIT SPECT Imaging in Parkinson’s Disease and Atypical Parkinsonian Syndromes
33.3.1 [123I]FP-CIT SPECT Imaging in Parkinson’s Disease
An important neuropathological characteristic of Parkinson’s disease (PD) is a severe loss of nigrostriatal dopamine neurons and consequently a decrease of striatal DATs (Kish et al. 1988; Kaufman and Madras 1991).
Several studies have established that [123I]FP-CIT SPECT scanning can detect a loss of striatal DAT binding even in the early motor phase of PD (Eshuis et al. 2009; Isaias et al. 2007; Booij et al. 1997a; Tissingh et al. 1998). Typically, the reduction in striatal DAT binding is stronger in the putamen than in the caudate nucleus and has an asymmetrical pattern (Fig. 33.2). Most often, DAT binding is lowest in the contralateral putamen (i.e. the side contralateral to the most affected side of the body). However, in individual PD patients, the decrease in DAT binding can be rather symmetrical or even lower on the ipsilateral than on the contralateral side. It has been suggested that this may be particularly true for PD patients with tremor-dominant features (Isaias et al. 2007). Interestingly, in many patients with clinically unilateral PD, there is a bilateral loss of putamen DAT binding (Tissingh et al. 1998; Filippi et al. 2005), which is consistent with studies in hemi-PD patients using [123I]β-CIT as a ligand (Marek et al. 1996) (Fig. 33.2). However, for routine clinical studies, it is important to be aware of the fact that in some patients with unilateral PD, the loss of DAT binding may be restricted to one side of the brain (Fig. 33.3).

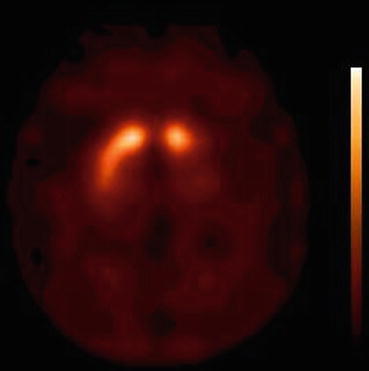

Fig. 33.2
[123I]FP-CIT SPECT scan (3 h after injection) of a hemi-parkinsonian patient. Note the asymmetry of binding, as well as the severe loss of binding bilaterally in the putamen

Fig. 33.3
[123I]FP-CIT SPECT scan (3 h after injection) of an early-stage PD patient. Note that in this hemi-parkinsonian patient (motor signs only at the right side of the body), the loss of striatal binding is restricted to the contralateral (left) striatum
In early-stage PD patients, using age-corrected data, an approximately 60–65 % loss of DAT availability can be found in the contralateral putamen (Booij et al. 2001; Tissingh et al; 1998). Additionally, recent studies have suggested that [123I]FP-CIT SPECT scanning is sensitive enough to detect a loss of striatal DAT binding in the premotor phase of PD (Iranzo et al. 2010; Stiasny-Kolster et al. 2005), consisted with findings from earlier [123I]β-CIT SPECT studies (Berendse et al. 2001; Ponsen et al. 2004; Ponsen et al. 2010). Finally, the loss of striatal [123I]FP-CIT binding in PD correlates with the degree of rigidity and hypokinesia, but not with the severity of resting or postural tremor (Spiegel et al. 2007; Isaias et al. 2007).
Although, in most studies in PD, loss of striatal DAT binding does not correlate with tremor scores (Spiegel et al. 2007; Isaias et al. 2007), it has however been suggested that a widespread degeneration of the nigrostriatal dopaminergic pathway might still play a role in the aetiology of rest tremor in PD (Isaias et al. 2007). Interestingly, tremor-dominant PD patients can have significantly lower DAT binding in the striatum ipsilateral to the rest tremor as compared to akinetic-rigid PD patients (Isaias et al. 2007). Moreover, in some patients with a strictly unilateral resting tremor (without bradykinesia and rigidity), reduced DAT binding may be restricted to the ipsilateral (instead of the contralateral) side of the striatum (Aguirregomozcorta et al. 2013; Lin and Chang 1997; Spiegel et al. 2007). These observations may be explained by degeneration/damage of “crossed” dopaminergic fibres (Aguirregomozcorta et al. 2013).
In some large clinical trials into which patients were enrolled based on their clinical diagnosis of PD, 11–15 % of patients showed no degeneration of the nigrostriatal pathway. In the ELLDOPA study, 15 % of the included PD patients who were scanned with [123I]β-CIT SPECT had normal baseline scans (Parkinson study group 2002). Importantly, the follow-up scans remained normal in the patients who were rescanned 4 year later. These patients have since been referred to as scans without evidence of dopaminergic deficit (SWEDDS; Fahn 2005). Recent studies suggest that many of these patients do not suffer from PD but from other conditions including dystonic tremor, parkinsonism associated with fragile X mental retardation 1 gene expansions or even non-neurological diseases (Bajaj et al. 2010; Hall et al. 2010; Schneider et al. 2007; Sixel-Döring et al. 2011). These data emphasise the diagnostic power of DAT imaging.
In routine clinical studies, [123I]FP-CIT SPECT is commonly used in inconclusive cases to differentiate PD from syndromes not characterised by nigrostriatal loss, e.g. essential tremor or drug-induced parkinsonism (Booij et al. 2001; Benamer et al. 2000; Marshall and Grosset 2003). In particular, the differentiation of PD from drug-induced parkinsonism (DIP) may present a diagnostic challenge to clinicians. Recent studies have shown that in this clinical situation, an abnormal [123I]FP-CIT SPECT scan is indicative of an underlying neurodegenerative process as is common in PD (Bovi et al. 2010; Diaz-Corrales et al. 2010; Kägi et al. 2010; Tinazzi et al. 2009; Lim et al. 2013). DIP may persist beyond 6–9 months from the time of neuroleptic withdrawal, as was demonstrated in a recent report on two patients in whom DAT binding was normal (Lim et al. 2013). Importantly, for an accurate interpretation of [123I]FP-CIT SPECT studies, it is not necessary to withdraw neuroleptics prior to the scanning procedure (Booij and Kemp 2008; Booij et al. 2010).
In summary, these findings suggest that [123I]FP-CIT SPECT is a sensitive means to detect a loss of striatal DAT binding in PD. In routine clinical studies, [123I]FP-CIT SPECT can be used to differentiate PD from other forms of parkinsonism that are not characterised by a loss of presynaptic dopaminergic neurons (e.g. essential tremor, psychogenic parkinsonism or drug-induced parkinsonism), in particular in clinically uncertain cases (Booij et al. 2001; Løkkegaard et al. 2002; Catafau et al. 2004; Cummings et al. 2011; Kägi et al. 2010; Marshall and Grosset 2003; Marshall et al. 2009).
33.3.2 [123I]FP-CIT SPECT Imaging in Multiple System Atrophy and Progressive Supranuclear Palsy
Multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) are neurodegenerative disorders associated with parkinsonism that are also characterised neuropathologically by a loss of nigrostriatal cells and consequently a loss of DATs (Kish et al. 1985; Ruberg et al. 1985; Warren et al. 2007; González et al. 2000).
[123I]FP-CIT SPECT has been used in several studies to demonstrate a loss of striatal DATs in MSA-P (MSA with predominantly parkinsonian signs) and PSP versus healthy controls (Antonini et al. 2003; El Fakhri et al. 2006; Plotkin et al. 2005; van Laere et al. 2006). Generally speaking, the results obtained using [123I]FP-CIT as a ligand in MSA-P are in line with findings in studies using [123I]β-CIT (Scherfler et al. 2005; Knudsen et al. 2004; Varrone et al. 2001; Seppi et al. 2006). In a recent prospective study, [123I]FP-CIT SPECT scanning was sensitive enough to detect MSA-P in the premotor phase (Iranzo et al. 2010). A subject with rapid eye movement sleep behaviour disorder but without parkinsonian signs had an abnormal [123I]FP-CIT SPECT scan and developed MSA-P 2.5 years later.
Even in MSA-C (MSA-cerebellar subtype) patients without parkinsonian signs, a significant loss of striatal DAT binding compared to controls has recently been demonstrated using [123I]FP-CIT SPECT (Muñoz et al. 2011). However, in comparison to patients with PD, MSA-P or PSP patients, the reported decrease in striatal DAT binding was relatively mild (an average loss of 20 % of putamen DAT binding). Interestingly, the results of this recent SPECT study suggest that most but not all MSA-C patients without parkinsonism do have some degree of nigrostriatal dopaminergic denervation. The observation that a loss of DAT binding does not occur in all MSA-C cases was confirmed by a recent post-mortem study in a pathologically confirmed MSA-C case (Kolenc et al. 2012).
In most MSA-P and PSP patients, DAT binding in the putamen is lower than in the caudate nucleus. However, particularly in PSP, some studies suggested a more uniform loss of DATs in the putamen and caudate nucleus (Antonini et al. 2003), but this could not be reproduced by others (Seppi et al. 2006). Furthermore, some studies have shown that striatal DAT binding may differentiate MSA and/or PSP patients from PD patients at a group level (Goebel et al. 2011; Stoffers et al. 2005), although this is not a consistent finding in SPECT studies (Seppi et al. 2006). In any case, there is considerable overlap in striatal DAT binding values between groups, which suggests that in a routine clinical setting DAT imaging cannot be used to differentiate PD from MSA-P or PSP in individual cases (Stoffers et al. 2005). A recent post-mortem study of PD and MSA-P cases confirmed that the loss of striatal DAT was not significantly different between MSA and PD patients and also that the asymmetry of DAT binding was not significantly different between the two groups of patients (Perju-Dumbrava et al. 2012).
In summary, the results of the above-discussed studies suggest that [123I]FP-CIT SPECT is a sensitive means to detect a loss of striatal DAT in MSA-P and PSP. However, it is important to emphasise that DAT imaging cannot be used to differentiate individual PD patients from MSA-P or PSP patients. Furthermore, striatal [123I]FP-CIT binding can be normal in some MSA-C patients.
33.3.3 [123I]FP-CIT SPECT Imaging in Corticobasal Degeneration
Corticobasal degeneration (CBD) is a rare syndrome, also characterised by nigrostriatal degeneration at autopsy (Oyanagi et al. 2001; Dickson et al. 2002). Indeed, an [123I]FP-CT SPECT that included the largest cohort of patients with a clinical diagnosis of probable CBD showed a loss of striatal DAT binding in 32 out of the 36 included patients (Cilia et al. 2011). In line with this observation, [123I]β-CIT SPECT and other [123I]FP-CIT SPECT studies in smaller groups of patients have also shown a loss of striatal DAT binding in CBD (Klaffke et al. 2006; Plotkin et al 2005).
Intriguingly, Cilia and co-workers (2011) demonstrated that the loss of striatal [123I]FP-CIT binding in their sample of CBD patients was characterised by a larger variability, more uniform striatal reduction and a greater asymmetry of binding than in PD patients. Indeed, DAT SPECT images in some CBD patients may be quite characteristic for CBD (Fig. 33.4), whereas in many other CBD cases the [123I]FP-CIT SPECT images may look similar to the images obtained in PD patients. Even of more interest, in some sporadic autopsy-proven CBD cases, an ante-mortem [123I]FP-CIT SPECT scan showed no evidence of a reduction in striatal DAT binding (O’Sullivan et al. 2008; Walker et al. 2002). In the study by Cilia and co-workers (2011), there were no remarkable differences in clinical or neuropsychological characteristics between CBD subjects with or without abnormal striatal [123I]FP-CIT binding. Apparently, extrapyramidal motor symptoms in CBD may not always be associated with nigrostriatal degeneration and could involve supranigral factors (Cilia et al. 2011).
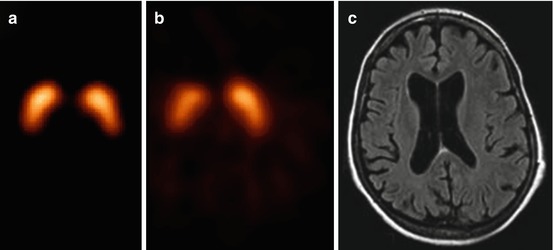

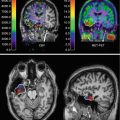
Fig. 33.4
[123I]β-CIT SPECT scan of a healthy control (a) and a patient suffering from corticobasal degeneration (b), as well as an MR image of the same patient (c). The corticobasal degeneration has led to an asymmetrical degeneration of the nigrostriatal pathway (b) and a unilateral atrophy of the brain (c), both contralateral to the body side that showed clinical motor signs (Reprinted from “Bewegingsstoornissen”; editors ECh Wolters and T van Laar, Amsterdam, 2002, The Netherlands)
For routine clinical studies, it is important to underline that in many, but not all CBD cases, an [123I]FP-CIT SPECT scan is abnormal. In some cases, the pattern of striatal DAT loss may even be characteristic for CBD (Fig. 33.4).
33.3.4 [123I]FP-CIT SPECT Imaging in Vascular Parkinsonism
Significant vascular lesions in the striatum or in the substantia nigra pars compacta can be associated with a loss of striatal [123I]FP-CIT binding (Marshall and Grosset 2003; Zijlmans et al 2002), although initial [123I]β-CIT SPECT studies suggested that the vast majority of patients suffering from vascular parkinsonism had a normal [123I]β-CIT SPECT scan (Gerschlager et al. 2002). In a hallmark paper by Zijlmans and co-workers (Zijlmans et al. 2007), even patients fulfilling clinical criteria for vascular parkinsonism and without vascular lesions in the striatum could have an abnormal [123I]FP-CIT SPECT scan, with bilateral loss of DAT binding, particularly in the putamen. A recent [123I]FP-CIT SPECT study in a large cohort of PD patients and patients with vascular parkinsonism confirmed these findings and showed that around 60–70 % of SPECT scans were abnormal in vascular parkinsonism (Benítez-Rivero et al. 2013). Importantly, scores based on visual pattern recognition discriminated better between PD and vascular parkinsonism cases than semi-quantitative scores based on the positioning of regions of interest. Lastly, in another recent [123I]FP-CIT SPECT study in PD patients and patients with vascular parkinsonism, a significant number of patients with vascular lesions in the basal ganglia had a normal SPECT scan, which indicates that vascular lesions in the basal ganglia do not always cause abnormal striatal DAT binding (Antonini et al. 2012). In the same study, a normal [123I]FP-CIT SPECT scan was associated with a lack of benefit from dopaminomimetic treatment in over 90 % of these subjects (Antonini et al. 2012).
All in all, a substantial number of patients with vascular parkinsonism have an abnormal [123I]FP-CIT SPECT. In this group of patients, accurate assessments of the images (including structural images), including careful visual inspection, may be of utmost importance to differentiate between vascular parkinsonism and degenerative diseases like PD.
33.3.5 [123I]FP-CIT SPECT Imaging in DLB
Like other diseases characterised by Lewy body pathology (i.e. PD), also dementia with Lewy bodies (DLB) is associated with a loss of dopamine neurons and striatal DATs (Perry et al. 1998). By contrast, the loss of striatal DATs may be only subtle in patients suffering from Alzheimer’s disease (Perry et al. 1998). In line with these autopsy findings, hallmark studies by Walker and colleagues demonstrated that ante-mortem DAT imaging with [123I]FP-CIT SPECT substantially enhanced the accuracy of a diagnosis of DLB by comparison with clinical criteria alone (Walker et al. 2002 and 2007). Many subsequent [123I]FP-CIT SPECT studies confirmed the loss of striatal DATs in DLB (Auning et al. 2011; McKeith et al. 2007; Morgan et al. 2012; Roselli et al. 2009; van Laere et al. 2006; O’Brien et al. 2004). [123I]FP-CIT SPECT may even detect a loss of striatal DAT binding in the preclinical phase of DLB (Iranzo et al. 2010). Importantly, the results of [123I]FP-CIT SPECT imaging may predict which patients with a clinical diagnosis of possible DLB will convert to probable DLB or to Alzheimer’s disease (O’Brien et al. 2009). Finally, a recent meta-analysis indicated that the diagnostic accuracy of [123I]FP-CIT SPECT in the diagnosis of DLB is high (Papathanasiou et al. 2012).
In most DLB patients, loss of striatal DAT binding is stronger in the putamen than in the caudate nucleus. Also, the loss of striatal DAT binding can be asymmetrical. However, in line with autopsy findings (Perry et al. 1998), the loss of striatal [123I]FP-CIT binding can also be symmetrical with a relative strong involvement of the caudate nucleus as compared to PD cases (Walker et al. 2004) (Fig. 33.5).
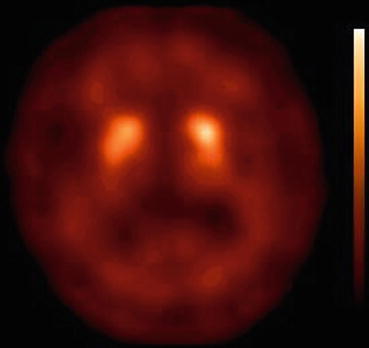

Fig. 33.5
[123I]FP-CIT SPECT scan obtained (3 h after injection) in a DLB patient. Note that due to the strong reduction in specific binding of [123I]FP-CIT in the striatum, the contrast between specific and non-specific binding is low. Also, the loss of binding is relatively symmetrical and involves both the caudate nucleus and putamen
Although [123I]FP-CIT SPECT appears to be very sensitive in differentiating between DLB and Alzheimer’s disease, the role in the differential diagnosis between PD with dementia (PDD), frontotemporal dementia and DLB is probably limited, since a recent study showed that a third of frontotemporal dementia cases had an abnormal [123I]FP-CIT SPECT scan (Morgan et al. 2012). Similarly, although at a group level loss of striatal DATs may be greater in PDD than DLB, the abnormalities on individual [123I]FP-CIT SPECT scans (i.e. lower DAT binding in the putamen than caudate nucleus) in PDD may be similar to the abnormalities observed in DLB (O’Brien et al. 2004; Colloby et al. 2012).
Importantly, a recent autopsy study in DLB, PDD and Alzheimer’s cases showed that ante-mortem DAT imaging was associated with post-mortem nigral dopaminergic neuronal density (but not α-synuclein, tau or amyloid burden) in demented patients (Colloby et al. 2012). This observation may cast doubt on the potential of DAT imaging to differentiate DLB patients without parkinsonism from Alzheimer’s disease. Nevertheless, most DLB patients without parkinsonism have abnormal DAT SPECT scans (O’Brien et al. 2004; McKeith et al. 2007). On the other hand, Alzheimer’s disease patients with parkinsonism may have normal scans (McKeith et al. 2007; Ceravolo et al. 2004).
To summarise, there is evidence that [123I]FP-CIT SPECT is helpful in differentiating DLB from Alzheimer’s disease, but its role in differentiating DLB from frontotemporal dementia may be limited.
33.4 Methods to Analyse [123I]FP-CIT SPECT Studies in Routine Clinical Practice
In routine clinical practice, many departments of nuclear medicine evaluate [123I]FP-CIT SPECT scans by visual inspection only. Major reasons are a lack of age-matched control data and the fact that it is a simple way to analyse images. Although a visual qualitative analysis may seem inaccurate, this approach can in fact be very effective in excluding or confirming a loss of striatal DATs (Benamer et al. 2000; O’Brien et al. 2004; McKeith et al. 2007). An important advantage of visual analysis over a quantitative approach is that an abnormal pattern can be recognised rapidly. A recent study showed that a visual analysis approach was even more accurate in differentiating vascular parkinsonism from PD than a semi-quantitative approach (Benítez-Rivero et al. 2013). Moreover, the visual interpretation of [123I]FP-CIT SPECT images can be further improved by visual rating of parametric distribution volume ratio images (Meyer et al. 2011). However, a visual analysis of [123I]FP-CIT SPECT images may be less accurate in patients in which the degeneration is quite symmetrical and the involvement of the caudate nucleus and putamen more uniform. This type of pattern has been described in some DLB, PSP and MSA-C patients.
Even though visual assessment of [123I]FP-CIT SPECT images remains an integral part of the study report, it is nowadays frequently supplemented by a quantification method (Tatsch and Poepperl 2012; Badiavas et al. 2011). Quantification does not only offer a more objective adjunct to an individual subjective judgement but also has the potential to detect subtle changes that can elude the human eye (Tatsch and Poepperl, 2012). In routine clinical DAT SPECT studies, semi-quantitative techniques using ROIs are commonly used due to its simplicity. Striatal ROIs were initially defined by manually drawing an irregular contour around the caudate nucleus and putamen and around a region devoid of DATs (occipital cortex or cerebellum). However, this method is strongly influenced by the intensity of striatal DAT binding. In other words, if there is decreased striatal FP-CIT binding (e.g. in a PD case), the reader tends to draw smaller ROIs over striatal regions than in cases with more intense striatal FP-CIT binding. Therefore, fixed (or predefined) ROIs are now used more frequently. These ROIs are derived from anatomical atlases or MR images and have led to a better standardisation. However, this technique is still investigator dependent and does not take into account interindividual differences in striatal volumes. From this perspective, it is of interest that several (semi)automated quantification tools have been developed and several methods have been proposed to quantify DAT studies by (semi)automated positioning of ROIs or volumes of interest (VOIs) (Badiavas et al. 2011; Habraken et al. 1999). Examples are BasGan (Calvini et al. 2007) and BRASS (Radau et al. 2000) (for a comprehensive review, see Tatsch and Poepperl 2012). Voxel-based analysis, e.g. using statistical parametric mapping (SPM), has also been used to analyse [123I]FP-CIT SPECT studies (Kas et al. 2007; van de Giessen et al. 2013). Automated voxel-based image analyses may increase the diagnostic accuracy of DAT imaging in differentiating between PD and other parkinsonian syndromes (Goebel et al. 2011). However, future studies are needed to evaluate the feasibility of voxel-based techniques in routine clinical practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree