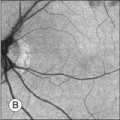
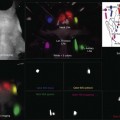
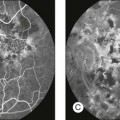
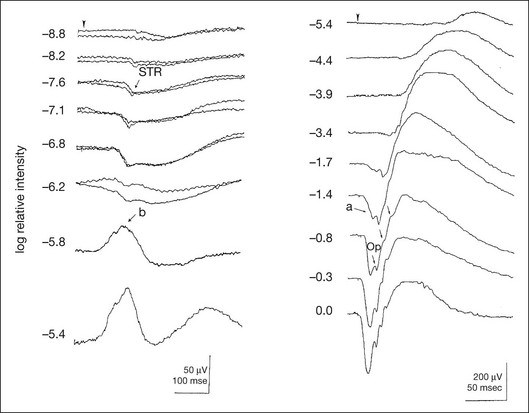
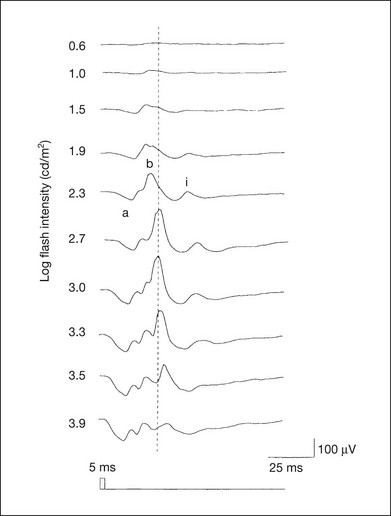
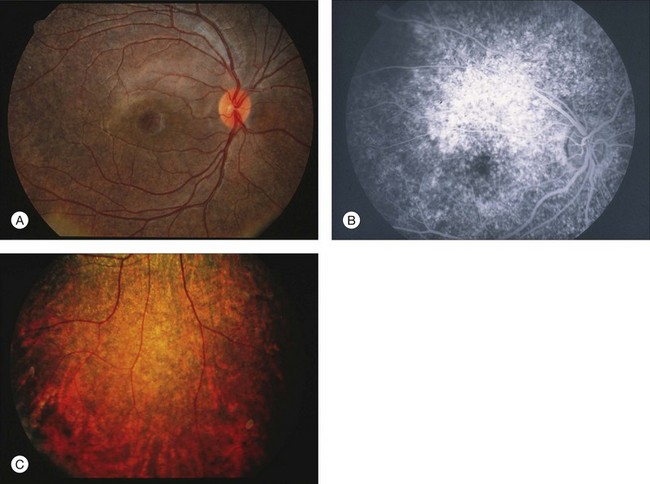
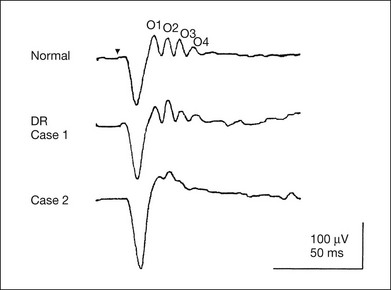
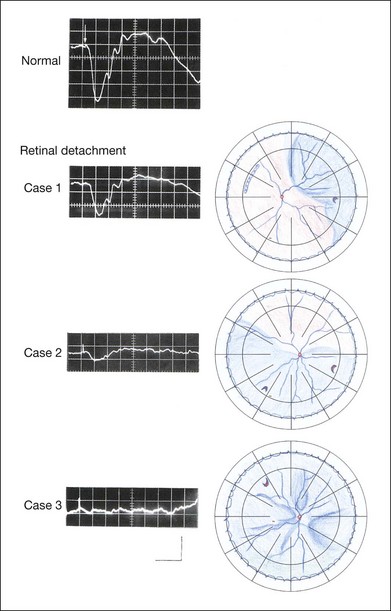
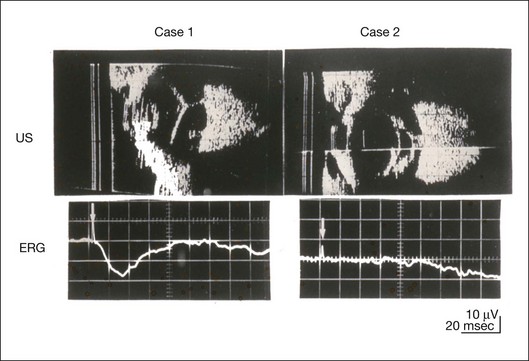
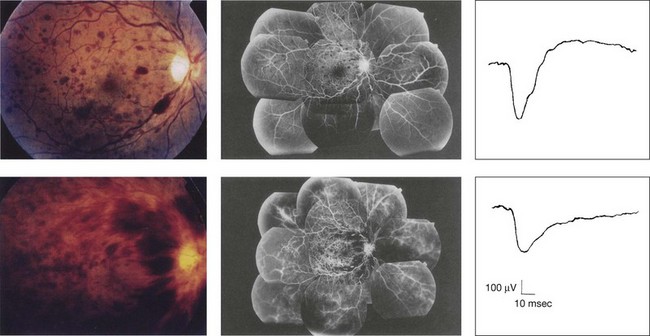
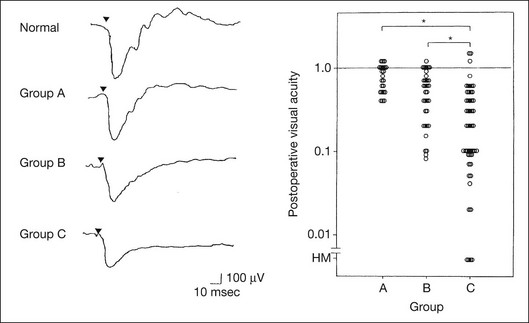
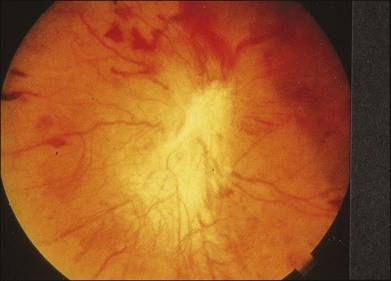
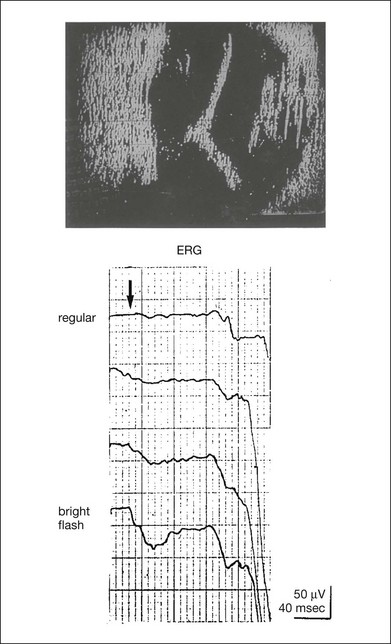
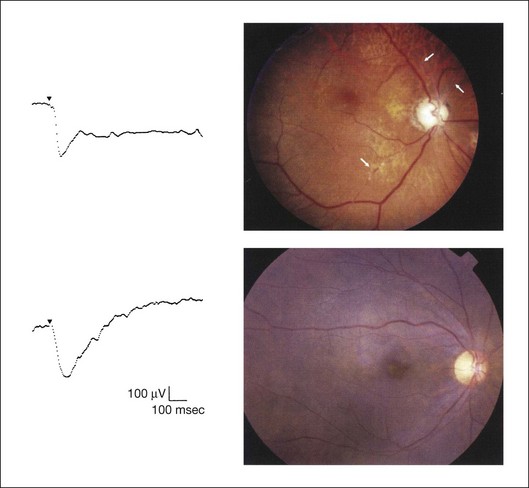
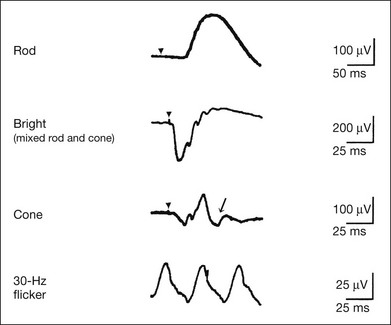
Chapter 8 Stimulus and recording devices The human ERG recorded at the cornea and elicited by a full-field stimulus is a mass response generated by cells across the entire retina. To obtain reproducible amplitudes and implicit times in the response, the stimulus and background light should be homogeneous and cover the entire retina, so all of the receptors are stimulated or adapted in a relatively homogeneous manner. The full-field, or Ganzfeld, stimulator represents such a stimulus. It consists of a large-diameter (40-cm) hemispheric dome (see Chapter 7, Electrogenesis of the electroretinogram) with a xenon stroboscopic light bulb placed at the top of the dome. This stimulus system has been recommended by the International Society of Clinical Electrophysiology for Vision (ISCEV) Standards Committee1 for use when obtaining clinical ERG recordings internationally. The ERG is recorded using corneal electrodes, usually referred to surface reference electrodes at the ipsilateral outer canthi or zygomatic fossae. Electrodes in common use2 include the Burian–Allen and the ERG jet, both of which are contact lens electrodes, the gold foil, Dawson–Trick–Litzkow (DTL), and H–K loop electrodes, which are noncontact lens. The representative electrodes are shown in Fig. 8.1. Fig. 8.1 Representative electroretinogram recording electrodes. Top, Burian–Allen electrode. A contact lens-type electrode with a lid speculum to minimize the effect of blinking and eyelid closure. Bottom, Dawson–Trick–Litzkow (DTL) electrode – a conductive Mylar thread usually placed in the lower fornix, it contacts the inferior bulbar conjunctiva or the corneal limbus. Dots show 10-mm distance. Figure 8.2 shows the full-field ERGs elicited by increasing stimulus intensities from a normal subject after 1 hour of dark adaptation. The ERGs elicited by relatively weak stimulus intensities are shown at the left and those by stronger stimulus are shown at the right. The calibrations for the amplitude and time are different for the weak and strong stimulus ERGs. The maximum stimulus luminance (0 log unit) is 44.2 cd/m2/s. Fig. 8.2 The full-field electroretinogram (ERG) elicited by increasing stimulus intensities recorded from a normal subject after 1 hour of dark adaptation. The left column shows responses elicited by relative low intensity and the right by relative high intensity. Note that the calibration differs for the ERGs in the two columns. Arrowheads indicate the stimulus onset. STR, scotopic threshold response; b, b-wave; a, a-wave; Op, oscillatory potentials. (Reproduced with permission from Miyake Y, Horiguchi M, Terasaki H, et al. Invest Ophthalmol Vis Sci 1994;35:3770–5.) At the left, the scotopic threshold response (STR),3 a cornea-negative wave, is first recorded at –8.2 log units, approximately 0.6 log units higher than psychophysical threshold. The maximum amplitude of STR is approximately 20 µV before it is masked by the developing b-wave. The implicit time of the STR near threshold is approximately 160 ms, and the implicit time decreases as the stimulus intensity increases. The STR originates from retinal neurons that are postsynaptic to the photoreceptors (see Chapter 7, Electrogenesis of the electroretinogram). As shown fully in Chapter 7, Electrogenesis of the ERG, many studies have shown that the a-wave of the full-field ERGs recorded in the dark is the leading edge of the photoreceptor potential.4 The b-wave originates indirectly from bipolar and Müller cells in the middle layers of the retina.5 The OPs are seen as a series of three or four rhythmic wavelets having almost equal intervals of about 6.5 ms in humans.6 The best experimental evidence indicates that the OPs reflect the activity of feedback synaptic circuits within the retina and represent an inhibitory or modulating effect of amacrine cells on the b-wave.7 The photopic, short-flash ERGs elicited by increasing stimulus intensities in a normal subject are shown in Fig. 8.3.8 At lower stimulus intensities, the amplitude of the b-wave increases with increasing stimulus until it reaches a maximum at a stimulus intensity of 3.0 log cd/m2. Further increases in the stimulus intensity result in a progressive decrease in the amplitude of the b-wave. Because a plot of the b-wave amplitude as a function of the stimulus intensity has an inverted U shape, this phenomenon has been termed the photopic hill phenomenon.9. Fig. 8.3 Photopic short-flash electroretinograms elicited by various stimulus intensities from a normal subject. Stimulus duration is 5 ms and the constant background illumination is 40 cd/m2. The vertical dashed line indicates 30 ms. The b-wave amplitude increases with increasing stimulus intensity until 3.0 log cd/m2. It decreases with further increases in stimulus intensity. When the b-wave amplitude is plotted against stimulus intensity, it shows an inverted U shape; this phenomenon has been termed the photopic hill phenomenon. (Reproduced with permission from Kondo M, Piao CH, Tanikawa A, et al. Japanese Journal of Ophthalmology 2000;44:20–8.) ERG recorded with a bright flash of light after dark adaptation for 30 minutes or longer (0 log unit in Fig. 8.2) shows mixed rod–cone response, which can provide variable information about retinal pathology and is of significant diagnostic value. We have an impression that about 70% of ERG information can be obtained by the evaluation of only the mixed rod–cone ERG. The five different types of mixed rod–cone ERG are shown in Fig. 8.4. Fig. 8.4 The five different types of mixed rod–cone electroretinogram (ERG). OP(–), selective reduction of oscillatory potentials; subnormal, both a- and b-waves are attenuated approximately to the same degree; negative, the amplitude of the b-wave is smaller than that of the a-wave; extinct, no discernible a- or b-wave. The normal type shows a-wave, b-wave, and OPs. The amplitude of b-wave is always larger than that of a-wave in the regular stimulus intensity range. The normal ERG can be seen in patients with localized macular dysfunction, optic nerve diseases, and central nervous system disease such as amblyopia. Even when the entire retina is ophthalmoscopically abnormal, such as rubella retinopathy or female carrier of ocular albino and choroideremia (Fig. 8.5), the ERG can be essentially normal. Fig. 8.5 Fundus photograph (A) and fluorescein angiogram (B) obtained from a 20-year-old man with rubella retinitis. Fundus photograph from a 60-year-old female carrier of choroideremia (C). The electroretinograms in these two patients were normal. (Reproduced with permission from Miyake Y. Electrodiagnosis of retinal diseases. Tokyo: Springer-Verlag; 2006.) An OP abnormality means either reduction of amplitude or delay of implicit time, or both. A selective OP abnormality is observed in the early stage of diabetic retinopathy10,11 (Fig. 8.6) or mild circulatory disturbance of retina such as central retinal vein occlusion. Fig. 8.6 Oscillatory potentials (OPs) of full-field electroretinograms recorded from a normal subject (top) and two patients with diabetic retinopathy (cases 1 and 2). The oscillatory potentials were found to have delayed implicit time (case 1) or reduced amplitude (case 2). DR, diabetic retinopathy. (Reproduced with permission from Miyake Y. Electrodiagnosis of retinal diseases. Tokyo: Springer-Verlag; 2006.) The amplitudes of all components are reduced approximately to the same degree. A reduced a-wave indicates abnormal photoreceptor function. This pattern is seen in patients with localized damage of the photoreceptors, such as partial retinal detachment or sectoral retinal degeneration. The amplitude of the full-field ERG is proportional to the area of functioning retina. This rule is shown when the extent of retinal detachment is compared with the ERG (Fig. 8.7). Fig. 8.7 Mixed rod–cone (bright flash) electroretinograms (left) and fundus drawings of three patients with rhegmatogenous retinal detachment (right). The reduction of the electroretinogram amplitude corresponds proportionally to the extent of retinal detachment. This rule is also shown in eyes with panretinal photocoagulation (PRP) in diabetic retinopathy. Following PRP, the amplitudes of ERG components are reduced by 40–45%, but the b-wave : a-wave (b/a) ratio is not changed significantly.11 When the media is hazy due to vitreous hemorrhage and the fundus is invisible, the presence or absence of retinal detachment is an important evaluation preoperatively. By combining ERG and ultrasonography, the differentiation between totally detached retina and dense vitreous membrane may be possible, as shown in Fig. 8.8. When the ERG is recordable, even if the amplitude is small, the thick membrane in the vitreous cavity is not totally detached retina, but vitreous membrane. Fig. 8.8 Ultrasonographic (US) image (top) and mixed rod–cone electroretinogram (ERG) (bottom) from eyes with vitreous hemorrhage. Ultrasonography shows thick membrane-like reflex in the vitreous cavity in both eyes. When the ERG is recordable, even if the amplitude is small, the thick membrane in the vitreous cavity is not totally detached retina, but vitreous membrane (case 1). In contrast, when the ERG is unrecordable, the thick membrane is most likely totally detached retina (case 2). Prognostic value: Among the acquired retinal diseases, the negative ERG may be seen in severe retinal circulatory disturbance such as central retinal arterial occlusion or proliferative diabetic retinopathy. In central retinal vein occlusion, the ischemic type shows negative ERG more frequently than nonischemic type, indicating that the b/a ratio can be an important index for evaluating the prognosis of central retinal vein occlusion.12,13 Figure 8.9 shows a patient with an initially normal, but later lower, b/a ratio which resulted in negative configuration in ERG.11 The fluorescein angiogram changed from the nonischemic pattern to the ischemic pattern, showing an extensive nonperfusion area accordingly. Fig. 8.9 Top panel, a 39-year-old woman had a central retinal vein occlusion in the right eye (top left). Fluorescein angiogram (top center) and electroretinogram (ERG) (top right) showed nonischemic pattern at her initial visit. Bottom panel, one month later, the retinal hemorrhage increased (bottom left), the fluorescein angiogram showed extensive areas of nonperfusion (bottom center), and the waveform of the ERG became negative (right). (Reproduced with permission from Miyake Y. Electrodiagnosis of retinal diseases. Tokyo: Springer-Verlag; 2006.) When massive vitreous hemorrhage prevents ophthalmoscopic examination of the fundus in patients with proliferative diabetic retinopathy, it makes it difficult to predict the surgical and visual outcome after vitrectomy. In these eyes, the amplitudes of the ERGs may be markedly reduced by various factors: pathological changes induced by the diabetic retinopathy, earlier PRP, and vitreous hemorrhage. As mentioned above, the PRP reduces the ERG amplitude without changing the b/a ratio.11 Because most diabetic patients with vitreous hemorrhage have undergone PRP, it is difficult to arrive at a prognosis of the outcome after vitrectomy using only the amplitudes. The b/a ratio provides more useful information about the visual prognosis after vitrectomy.14 The preoperative mixed rod–cone ERGs were classified into three groups in patients with diabetic retinopathy associated with significant vitreous hemorrhage (Fig. 8.10, left). Group A indicates those with a b/a ratio>1.0 and the OPs are clearly recordable. Group B includes those with a b/a ratio >1.0 but the OPs are absent. Group C comprises those with a b/a ratio <1.0 with absent OPs. Thick proliferative tissues were found at the disc (Fig. 8.11) intraoperatively in 36% of the eyes in group A, 67% in group B, and 90% in group C.14 It was suggested that the fibrous proliferation at the disc may restrict retinal circulation by compressing the central retinal artery. Fig. 8.10 Preoperative full-field electroretinograms (ERGs) recorded from a normal control and three diabetic patients with vitreous hemorrhage (HM) who were classified into three groups (left). Postoperative visual acuity in the three groups classified according to the ERG waveform (right), showing that the postoperative visual acuity for group C was significantly worse than that for group A or group B. (Reproduced with permission from Kondo M, Piao CH, Tanikawa A, et al. Japanese Journal of Ophthalmology 2000;44:20–8.) Fig. 8.11 Proliferative tissue on the optic disc in a patient with diabetic retinopathy. (Reproduced with permission from Miyake Y. Electrodiagnosis of retinal diseases. Tokyo: Springer-Verlag; 2006.) The distribution of the postoperative visual acuity for each group is shown in Fig. 8.10, right.14 The postoperative visual acuity for group C was significantly worse than for group A or group B. The low b/a ratio may indicate more severe ischemic retina, which in turn may account for the relatively good correlation with visual acuity. However, among the patients in group C, there were some whose postoperative visual acuity was good, indicating that a b/a ratio <1.0 is not necessarily a contraindication for vitrectomy. Another important finding is that most patients who have distinct OPs preoperatively have favorable visual acuity after vitrectomy. This observation is important when we discuss the visual prognosis with patients before surgery. The light-filtering effect of a dense vitreous hemorrhage should also be considered when evaluating the preoperative ERG in diabetic patients. Severe vitreous hemorrhage reduces the intensity of the stimulus light reaching the retina, which can increase the b/a ratio (Fig. 8.2). When the vitreous hemorrhage is extremely dense, the intensity of stimulus light may be decreased and the effective stimulus light to evoke ERG may not reach the retina. In such situations, we need a much brighter stimulus than the regular maximum stimulus to evoke ERG. Such an example is shown in Fig. 8.12. In such cases, we have an impression that ERG often has a negative configuration, as shown in this patient. Fig. 8.12 Ultrasonographic image (top) and mixed rod–cone electroretinograms (ERGs) (bottom) with various stimulus intensities from eyes with extremely dense vitreous hemorrhage. As the intensity of the stimulus light is decreased, a sufficiently bright stimulus to evoke the ERG may not reach the retina. In this situation, a much brighter stimulus than the regular maximum stimulus may evoke the ERG response. In such cases, it appears that the ERG frequently shows negative configuration. In the prognostic evaluation of eyes that develop endophthalmitis after intraocular lens implantation, the b/a ratio is also valuable.15 Eyes with early (within 1 week) endophthalmitis associated with a b/a ratio of <1.0 have a worse postoperative prognosis than eyes with late-onset endophthalmitis and/or a b/a ratio of >1.0. These observations are quite important when deciding on the appropriate time to perform vitrectomy for treatment. For example, a patient with endophthalmitis that was detected within 1 week of intraocular lens implantation and with an ERG b/a ratio of <1.0 should undergo vitrectomy urgently. On the other hand, when endophthalmitis develops a relatively long time after surgery and the ERG b/a ratio is >1.0, the timing of the vitrectomy is not as critical. Representative examples15 are shown in Fig. 8.13. Fig. 8.13 Left, Preoperative mixed rod–cone (bright flash) electroretinograms (ERGs) recorded from two patients with endophthalmitis after intraocular lens implantation. The negative configuration of the ERG (case 1) suggests poorer visual prognosis after vitrectomy than for the patient with normal-shaped ERGs (case 2). Right, postoperative fundus. Case 1 showed extensive retinal vascular occlusions (arrows), with poor postoperative visual function, as expected. Case 2 showed an essentially normal fundus with good postoperative visual function. (Reproduced with permission from Horio N, Terasaki H, Yamamoto E, et al. Am J Ophthalmol 2001;132:258–9.) Diagnostic value: The negative ERG is seen in some hereditary retinal diseases, which provides diagnostic information, particularly when the a-wave amplitude is normal. The representative diseases, where the negative ERG shows the diagnostic value, include complete-type congenital stationary night blindness16 (CSNB: see Fig. 8.20, below), incomplete-type CSNB16 (see Fig. 8.20, below), X-linked juvenile retinoschisis11 (XLRS: see Fig. 8.20), juvenile-onset neuronal ceroid lipofuscinosis,17 and infantile Refsum disease. Since both complete and incomplete CSNB show essentially normal fundi and most patients with CSNB have moderately low visual acuity,16 the negative ERG finding is extremely important to pick up these disorders, differentiating them from other diseases with normal fundi, low visual acuity, and normal ERG, such as psychological eye problems, amblyopia, optic nerve disease, central nervous system disease, or occult macular dystrophy (OMD). The detailed findings separating rod and cone components will be treated later. When the a-wave amplitude is normal and the b/a ratio is less than 1.0, the selective abnormality of the second-order neuron is indicated. On the other hand, when the amplitude of the a-wave is smaller than normal with the b/a ratio <1.0, there are two interpretations. One is the combined dysfunction of photoreceptor and middle retinal layer. This situation is often observed in patients with retinitis pigmentosa. The other is the ERG showing the photopic hill phenomenon8 (see above). When the rod function is completely gone and the cone function is well preserved, the ERG shows cone ERG even in the dark. In this condition, when the stimulus light intensity is strong, the ERG reveals the photopic hill phenomenon (Fig. 8.3), showing negative configuration with a small a-wave. Such examples are seen in bright flash mixed rod–cone ERG in the dark in Oguchi disease or fundus albipunctatus (FA) (Fig. 8.16). In acquired diseases, negative ERG may be seen in melanoma-associated retinopathy,18,19 birdshot choroidopathy,20 ocular siderosis, quinine retinopathy, and methanol toxicity. The negative configuration of ERG provides the diagnostic value in these disorders. The extinct ERG is often seen in the advanced stage of rod–cone dystrophy, including retinitis pigmentosa, gyrate atrophy or choroideremia, and total retinal detachment. In retinitis pigmentosa, gyrate atrophy, or choroideremia, even when the macular area is preserved, ERG may become undetectable. Cancer-associated retinopathy,19 an autoimmune retinopathy, may often show extinct ERG and should be differentiated from retinitis pigmentosa. Although the rods outnumber the cones 13 to 1 in the normal human retina, the cone ERG response accounts for 20–25% of the ERG response amplitude. For the purposes of diagnosis, it often becomes necessary for the examiner to evaluate rod and cone activity separately. The full-field ERGs using the ISCEV Standard (see Chapter 7, Electrogenesis of the electroretinogram) in a normal subject are shown in Fig. 8.14. Fig. 8.14 Standard full-field electroretinograms with isolation of the rod and cone components. Arrowheads indicate the stimulus onset. The arrow indicates photopic negative response. After 30 minutes of dark adaptation, a rod (scotopic) ERG is recorded with a dim flash of light at approximately –3.9 log units in Fig. 8.2. A bright flash (mixed rod–cone) ERG is elicited by a single flash of white light at maximum intensity of log 0 units in Fig. 8.2. Cone and 30-Hz flicker ERG are recorded with a stimulus intensity of 3.3 log units in Fig. 8.3 under the background illumination of 40 cd/m2, which is sufficient to suppress all rod activity. The photopic recordings (cone and 30-Hz flicker ERG) are made after 10 minutes of light adaptation to 40 cd/m2, because the maximum photopic ERG can be obtained when recorded after light adaptation.11 In addition to the conventional ERG components, the photopic negative response (PhNR) was introduced21: this originates from retinal ganglion cells and will be treated more fully later. The congenital stationary disorder of cone dysfunction is represented by rod monochromacy which is inherited in an autosomal recessive mode. This disorder is characterized in the complete form by complete absence or severely depressed color vision, reduced visual acuity, nystagmus, and photophobia11. There is also an incomplete form of this disorder, where color vision and/or visual acuity is not severely affected.11 In both forms, the fundus and fluorescein angiograms are normal, and the most characteristic feature in terms of the diagnosis is selective reduction or absence of the photopic components while preserving normal scotopic components of the full-field ERG even in incomplete form (Fig. 8.15). Molecular genetic studies have shown that mutations in the CNGB3 gene encoding the β-subunit of the cone photoreceptor cGMP-gated channel are responsible for rod monochromacy.22 Fig. 8.15 Full-field electroretinograms (ERGs) and Farnsworth dischromous panel D-15 test from patients with cone photoreceptor dysfunction. Blue cone monochromacy shares many characteristics with rod monochromacy, except that the hereditary mode is X-linked recessive11. The visual acuity is approximately 0.2–0.3, which is slightly better than that of the complete form of rod monochromacy. Unlike rod monochromacy, the blue cone function is selectively preserved. Panel D-15 test shows several crossing lines perpendicular to the tritan axis (Fig. 8.15). The fundus is essentially normal, although in the late stage some atrophic changes may develop in the macula. The molecular genetics study indicated that mutations exist in the red and green opsin in the blue cone monochromacy. The full-field ERGs are similar to those of rod monochromacy, showing nearly normal rod ERGs with absence of the photopic ERG (Fig. 8.15). Although the blue cone ERG is normally present, the amplitude of the normal blue cone ERG is too small to be detected in the regular full-field cone ERG, and the implicit time is too long to follow 30-Hz flicker ERG stimuli (see section on S-cone ERG, below). Oguchi disease, first reported by Oguchi23 in 1907, is an unusual form of CSNB with autosomal recessive inheritance. It is characterized by a peculiar grayish white discoloration of the fundus. This unusual fundus coloration disappears after a long period of dark adaptation, which is called the Mizuo–Nakamura phenomenon24. Only the rod function is abnormal and is absent after 30 minutes of dark adaptation, but the subjective and electroretinographic rod function may increase after 2–3 hours of dark adaptation.25 Mutations in the gene of arrestin26 or the rhodopsin kinase,27 both of which are rod phototransduction, cause the recessive form of Oguchi disease. Full-field ERGs (Fig. 8.16) recorded after 30 minutes of dark adaptation show absent rod ERG and essentially normal cone-mediated ERG.25
Clinical Electrophysiology
Standard full-field ERG
Stimulus intensity versus ERG responses and components
Photopic condition
Bright flash mixed rod–cone ERG
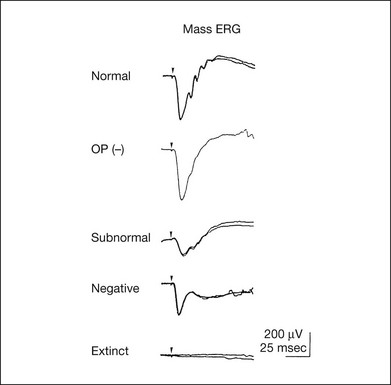
Normal
Selectively abnormal oscillatory potentials
Subnormal
Negative
Extinct
Isolation of rod and cone components in standardized ERG
Cone photoreceptor dysfunction
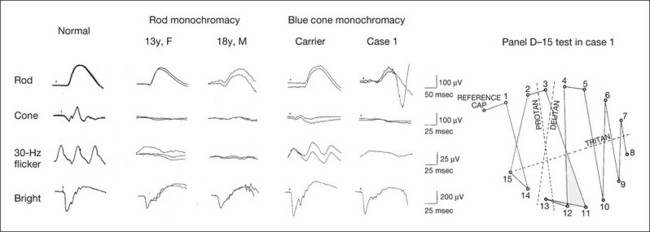
Second and third columns (from left), full-field ERGs recorded from two siblings with rod monochromacy showing selective absence of the photopic components. During 10-year follow-up, their visual function remained stable and their fundi remained normal. Visual acuity was 0.1/0.4 in a 13-year-old sister and 1.0/1.0 in an 18-year-old brother. The sister showed mild acquired red–green deficiency and the brother had normal color vision due to functional cones preserved only in the fovea.
Fourth and fifth (columns from left), full-field ERGs recorded from a family with blue cone monochromacy (carrier mother and son) showing normal rod components and nearly absent cone components. Although the blue cone ERG is normally present, the amplitude of the normal blue cone ERG is too small to be detected in the regular full-field cone ERG, and the implicit time is too long to follow 30-Hz flicker ERG stimuli.
Rightmost column, Farnsworth dischromous panel D-15 test from case 1 showing that several crossing lines were perpendicular to the tritan axis. (Revised and reproduced with permission from Kondo M, Piao CH, Tanikawa A, et al. Japanese Journal of Ophthalmology 2000;44:20–8, with permission.)
Rod photoreceptor dysfunction
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Clinical Electrophysiology