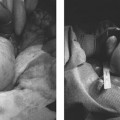
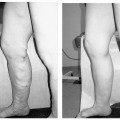
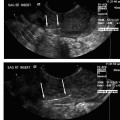
18 Clinical Perspective: Pelvic Pain (Gynecology) C. Paul Perry Chronic pelvic pain in women is a common and disabling condition. It is defined as pelvic pain, which has been present for 6 months or longer. Two to ten percent of all gynecologic office consultations are for chronic pelvic pain and 20% of all laparoscopic evaluations are performed for chronic pelvic pain. It is estimated that 10 million women suffer from this condition and that 7 million do not seek help. The economic impact of this condition is astonishing. The annual medical cost for diagnosis and treatment of chronic pelvic pain is estimated to be approximately $1.2 billion. The cost of lost productivity in these patients is estimated to be $15 billion annually. In contrast to episodic and acute pain, chronic pain is almost always continuous and unresponsive to conventional diagnosis and treatment. If unrelieved, it can potentially result in long-term disability, depression, and neurologic changes. Chronic pelvic pain (CPP) can be caused by numerous organic pathologies usually accompanied with variable psychologic dysfunctions. The most commonly made diagnosis in chronic pelvic pain is endometriosis (31%). After evaluation, up to 61% of patients are found to have no explanation for their pain. The majority remains undiagnosed or improperly diagnosed.1 Many improperly diagnosed patients will undergo misdirected therapy. These and other patients often abandon their pursuit of medical help due to the fact that they never receive an explanation or any treatment for their pain. The majority of women with no obvious pathologic cause for their pain may be suffering from pelvic congestion syndrome (PCS). Pelvic congestion is defined as the presence of enlarged venous complexes of the reproductive tissues with impaired circulation and drainage. This can produce pain alone or an association with other more common pathologies (e.g., endometriosis, adhesions, etc.). PCS is a diagnosis that was proposed over 100 years ago, but is only recently regaining some legitimacy. Richet is credited with first describing this condition as a case of “tubo-ovarian varicocele.”2 Before the days of laparoscopy and venography, Howard Taylor described this syndrome and gained some credibility in the medical community of the 1950s. However, due to assumptions regarding the psychosexual component of this illness and the fact that stress always aggravates the pain, many physicians saw this as a purely psychosomatic illness.3,4 The resurgence of pelvic congestion as a legitimate cause of chronic pelvic pain is due to the elegant and systematic work of Beard’s group at St. Mary’s Hospital in London.3 Starting in the 1970s and proceeding through the present, the clinical characteristics, methods and criteria for diagnosis, and psychological components have been addressed in the United Kingdom without totally being accepted by the American academic and medical practicing community. Until recently, gynecologists in the United States have viewed PCS as rare, psychosomatic, or imaginary. Our view is certainly evolving. Better clinical data with a clear association between symptoms, diagnostic criteria, and treatment outcomes has won the respect of many including the American College of Obstetrics and Gynecology.5 To this we and our patients owe a great debt to Dr. Richard Beard’s group in the United Kingdom.6 A recent prevalence study suggests that 9.9% of women will have radiologic evidence of pelvic varicosities and 59% of these patients will be symptomatic. The importance of this finding is supported by the fact that up to 77% of these symptomatic patients might benefit from therapy.7 Several possible etiologies for the venous congestion and pelvic pain associated with PCS have been classified by El-Minawi into categories including anatomic dysfunction, orgasmic dysfunction, hormonal dysfunction, psychosomatic dysfunction, and iatrogenic dysfunction.8 Unfortunately, these are not complete and do not singularly explain all the clinical findings of pelvic congestion, each failing in some respect. The rich anastomotic venous plexuses of the pelvic viscera include ovarian, paraovarian, uterine, bladder, rectal, and vulvar veins. The vulvar and uterine veins normally drain into the internal iliac veins. Anatomically, the left ovarian vein drains into the left renal vein and then into the inferior vena cava while the right ovarian vein drains directly into the inferior vena cava. Vascular connections exist between the bladder and rectal venous complexes as well with the veins of the upper thigh. These channels are relatively valveless and are gravity and vascular-tone dependent for their circulation. Anatomic studies have shown that 13 to 15% of women lack valves in the left ovarian vein; the corresponding figure for the right ovarian vein is 6%. When present, 43% of the valves on the left and 35 to 41% on the right are incompetent. Mean values of ovarian venous diameter are 3.8 mm in the presence of competent valves and 7.5 mm when the valves are incompetent due to resulting reflux and congestion within the vein. The upper limit of the normal diameter for ovarian veins is considered to be 5 mm.9 The structure of the human ovarian vein has been studied with transmission electron microscopy. This has revealed that the vein has typical venous endothelium that is immediately adjacent to the first of three smooth layers; no elastic lamina separates the endothelium from the inner layer of smooth muscle. Thinning of the inner circular layer of smooth muscle can be seen with advancing age.10 The middle smooth muscle layer consists of a circular arrangement of fibers. The outer layer of smooth muscle is arranged longitudinally with collagen and with autonomic nerves penetrating throughout the muscle. Anatomic dysfunction is thought by most to play a role in the development of PCS. Pelvic varicosities are thought to be due primarily to the effect of gravity on an incompetent venous valvular system. The resultant stasis produces the congestion and pain that is associated with this condition. Parity is a known risk factor in the development of PCS.11 It is known that pregnancy increases the capacity of the pelvic veins by 60%. When this is combined with the venous kinking of a malpositioned gravid uterus, venous stagnation, flow reversal, and pelvic varicosities are likely to occur. Allen and Master’s controversial theory regarding a “fascial defect” producing uterine malposition and congestion has been discounted.12 However, an additional anatomic relationship between retroversion of the uterus with pelvic pain and dilated pelvic veins has been established.13 Extensive cadaver dissections of pelvic venous vasculature have confirmed the scarcity of valves in these veins. These studies have led to the conclusion that genetic structural venous wall anomalies are a major contributing factor in the development of pelvic varicosities.14 The nutcracker syndrome, which is compression of the left renal vein by the superior mesenteric artery, can lead to high pressure within the left ovarian vein and pelvic varicosities.15–17 When a female patient is sexually stimulated up to, but not reaching, orgasm (the plateau phase of a woman’s sexual response), some pain caused by vasocongestion may be felt in the pelvic viscera. Whether or not this can produce permanent vascular changes remains unknown. In 1949, Taylor wrote that “psychiatric disturbances, usually of an emotional character, are a common accompaniment of pelvic congestion.”4 It was his opinion that an important factor in the etiology of pelvic congestion was “the effect of a primary state of emotional tension” on the smooth muscle and secretory cells of the pelvis in producing psychosomatic disturbances.4 Later studies seem to confirm psychosomatic contribution to pelvic pain in that women who suffered from chronic pelvic pain were psychologically different from women without pain. They tended to be more neurotic and to have abnormal attitudes toward their own and their partner’s sexuality.3 This caused pelvic congestion to be considered by some as a purely psychiatric condition (pelipathia vegetativa). Beard and colleagues partially confirmed this by finding that women with pelvic congestion tended to be more neurotic and were in less satisfying relationships.18 As El-Minawi points out, this proposition brings up the old adage regarding the “chicken and the egg.” It is true that these women will have some psychologic overlay, but we have learned that the stress of chronic pain itself induces many of these social and psychologic consequences. Women with pelvic congestion have a higher incidence of multicystic ovaries, enlarged uteri, and thickened endometrium, which are all findings that may be hormonally induced. On ultrasound, up to 56% of women with pelvic varicosities and pain were found to have polycystic or multicystic ovaries.19 Taylor and Beard mentioned the possibility that this condition might be related to hormonal sensitivity because it is virtually unknown in postmenopausal women. Reginald et al found that by inhibiting the effect of estrogen with medroxyprogesterone acetate (MPA), a decrease in the degree of pelvic congestion can be demonstrated on venography. In addition, the majority of these women experienced symptomatic relief when compared with controls.20 Tubal ligation procedures and the use of intrauterine devices for contraception have both been theorized to be associated with PCS. In one series, 60% of patients with pelvic congestion were found to have had undergone a tubal ligation procedure.21 These patients were also found to have a greater volume of peritoneal transudate with fluid containing higher levels of 6 keto-prostaglandin α than controls. These patients were symptomatic 16 to 39% of the time. An additional study demonstrated venographically (95%) and laparoscopically (52%) that there is an association between PCS and the Lippes loop.8 Larger studies have yet to verify any of these causes. Janicki has postulated that the pain and vasodilatation of pelvic congestion is actually a manifestation of a visceral complex regional pain syndrome.22 As nociceptive signals from any pelvic pain pathology are transmitted to the dorsal horn receptor cells of the spinal cord, antidromic transmission to pelvic vasculature produces venous changes. Endothelial release of neurotransmitters then produces the pain and tenderness associated with PCS. Stones et al have identified several neurotransmitters produced by the abnormal vessels seen in PCS, which may play a role in the pathogenesis of this syndrome. These include adenosine 5′ triphosphate, substance P, endothelin, vasopressin, calcitonin gene-related peptide and nitric oxide.23–26
Pathogenesis
Anatomic Dysfunction
Orgasmic Dysfunction
Psychosomatic Dysfunction
Hormonal Dysfunction
Iatrogenically Induced Dysfunction
Neuropathic Dysfunction
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree