15 Clinical Review: Pelvic Pain Jafar Golzarian, Fadi Youness, and Colleen M. Kennedy The definition of chronic pelvic pain is noncyclic pain of 6 or more months duration that localizes to the anatomic pelvis, abdominal wall at or below the umbilicus, lumbo-sacral back, or the buttocks and is of sufficient severity to cause functional disability or lead to medical care.1 Pelvic pain may further be categorized as acute (typically less than 3 months in duration), or chronic (pain lasting longer than 6 months in duration). Estimates suggest that 15 to 20% of women in the United States between the ages of 18 and 50 years have experienced chronic pelvic pain.2 Although chronic pelvic pain accounts for 10 to 40% of all outpatient gynecologic visits,3 it is important to note that the most frequent disorders associated with chronic pelvic pain are often nongynecologic, including irritable bowel syndrome, painful bladder syndrome, and musculoskeletal disorders.4 Patients younger than 35 years and white women are at higher risk of developing this condition. Chronic pelvic pain is responsible for 35% of diagnostic laparoscopies and 15% of all hysterectomies performed in the United States. Finally, this condition is associated with a substantial economic impact as manifested by work absenteeism and health care cost. It is estimated that the cost of care for women with chronic pelvic pain approaches $39 billion per year in the United States.1 Acute pelvic pain often warrants investigation, including laboratory and radiologic studies, to determine whether or not the etiology of pain necessitates an immediate intervention such as with appendicitis or a ruptured ectopic pregnancy. Although, patients with an acute surgical abdomen will often have a rapid onset of symptoms, patients with bowel obstruction necessitating emergent surgery may present with weeks of vague abdominal pain, followed by a sudden deterioration. The list of disorders causing pelvic pain is exhaustive; common etiologies of acute pelvic pain are noted in Table 15.1.5 In contrast to patients presenting with acute pelvic pain, women presenting for an evaluation of chronic pelvic pain have typically already undergone an extensive evaluation, which may or may not have revealed an etiology for their pain. They may have been seen by a variety of different medical specialists given the many nongynecologic conditions associated with chronic pelvic pain. However, up to 20% of patients remain symptomatic after undergoing multiple diagnostic and therapeutic procedures. This is a source of frustration and anxiety for patients with chronic pelvic pain. It is also increases the importance for any practitioner evaluating these patients to understand the extensive differential diagnosis that must be applied to these patients. Common conditions that may exacerbate or cause chronic pelvic pain are noted in Table 15.2.1 When a patient with pelvic pain is being evaluated, it is often tempting to attribute this to gynecologic causes, especially in the context of an interventional radiology practice evaluating patients prior to procedure such as uterine fibroid embolization (UFE) or ovarian vein embolization. However, it is important to remember that these patients may suffer from one of a large number of etiologies including gastrointestinal (GI), urologic, musculoskeletal, neurologic, psychologic, and vascular disorders among others. Therefore, a comprehensive history and physical examination are critically important parts of the evaluation of a patient with chronic pelvic pain.
Evaluating a Patient with Chronic Pelvic Pain
| Gynecologic |
| Ruptured ectopic pregnancy (rule out first!) |
| Endometriosis |
| Endometritis |
| Pelvic inflammatory disease |
| Leiomyomas (degeneration, infarction, torsion) |
| Ovarian cysts or masses with bleeding, torsion, or rupture |
| Gastrointestinal |
| Appendicitis |
| Diverticulitis |
| Colitis/ Ileitis (viral, bacterial, other) |
| Peritonitis |
| Bowel obstruction |
| Urologic |
| Bladder outlet obstruction |
| Cystitis |
| Renal lithiasis |
| Pyelonephritis |
| Vascular |
| Mesenteric ischemia/ infarction |
| Dissecting or ruptured aortic aneurysm |
| Bowel wall hematoma |
| Sickle cell disease |
| Gynecologic |
| Endometriosis |
| Pelvic congestion syndrome |
| Pelvic inflammatory disease |
| Ovarian retention syndrome |
| Ovarian remnant syndrome |
| Leiomyomas |
| Adenomyosis |
| Adhesions |
| Cervical stenosis |
| Pudendal nerve entrapment |
| Vulvodynia |
| Gastrointestinal |
| Colon cancer |
| Constipation |
| Inflammatory bowel disease |
| Irritable bowel syndrome |
| Urologic |
| Bladder cancer |
| Interstitial cystitis |
| Radiation cystitis |
| Urethral syndrome |
| Musculoskeletal |
| Abdominal wall myofascial pain |
| Chronic coccygeal or back pain |
| Fibromyalgia |
| Pelvic floor myalgia |
| Other |
| Depression |
| Hyperalgesia |
| Somatization disorder |
| Celiac disease |
| Porphyria |
| Shingles |
When obtaining a history from these patients, their obstetric, surgical, psychosocial, and sexual history must be covered. In addition, the characteristics of their pain (e.g., location, severity, quality, timing, and exacerbating and relieving factors) must be reviewed because they may point toward an etiology for their pain.7 If the patient has undergone previous therapy, the effect that these treatments have had on the pain must be reviewed. A complete review of systems is important due to the multifactorial etiology that is often present in patients with chronic pelvic pain. The goal of a physical examination is to detect the exact anatomic location of tenderness and to then correlate these findings with the location of the patient’s pain. The evaluation must cover the reproductive tract, in addition to the musculoskeletal, GI, urinary, and neurologic systems.8
Imaging studies will likely make up the next component of the evaluation of patients with chronic pelvic pain. The imaging tests ordered will be based on the most highly suspected etiology for the patient’s pain based on the above history and physical examination. Laparoscopy remains an important part of the diagnostic evaluation of these patients; more than one-third of diagnostic laparoscopies are done in this setting. Although conditions such as endometriosis and adhesions are often diagnosed with laparoscopy, it is important to remember that up to one-third of laparoscopic studies in women with chronic pelvic pain have normal findings. This, however, does not mean that a woman has no physical basis for her pain.7 In addition, pathology identified at laparoscopy may not be responsible for the patient’s pain.9–11
After a typically extensive evaluation has taken place, treatment options will be discussed with the patient. Some of the conditions that can potentially be addressed by interventional radiologists are reviewed in this chapter and others within this text. Other conditions highlighted in this chapter, though not likely to be treated by interventional radiologists, are ones that will likely already have been discussed with patients and will therefore come up during their evaluations of patients with chronic pelvic pain.
Differential Diagnosis
Endometriosis
The most common gynecologic diagnosis among women with chronic pelvic pain is endometriosis.12 Although 30% of women evaluated for chronic pain in the general population are found to have endometriosis, over 70% of women were given a diagnosis of endometriosis when seen in a gynecology specialty practice.12 Despite the fact that interventional radiologists may not themselves be directly involved in treating these patients, it is important to be aware of this condition when evaluating patients with chronic pelvic pain for procedures such as ovarian vein embolization or uterine artery embolization.
Endometriosis is classically described as the presence of endometrial-like tissue (glands and stroma) outside the uterine cavity and musculature.13 The etiology of this remains unknown, but theories do exist including coelomic metaplasia, lymphatic or hematologic spread, or implantation of cells carried in retrograde menstrual flow. Retrograde menstruation can seed the peritoneal cavity with endometrial cells, which leads to stimulated angiogenesis and lesion development.13,14 This can lead to an intraperitoneal cascade of cytokines and other factors that result in the pain associated with endometriosis.15,16 However, because retrograde menstruation can occur in up to 90% of normal patients, it is clear that other factors are involved in the pathogenesis of endometriosis.17
A history including pelvic pain, dysmenorrhea, dyspareunia, and infertility is suggestive of endometriosis. The pain associated with endometriosis is generally cyclical, although it may become continuous as the disease worsens.18,19 Lesions involving the bladder or rectum can cause pain during urination or defecation.18,20 Endometriosis is also associated with infertility, especially when there is advanced disease due to the distortion of normal pelvic anatomy and impairment of tuboovarian function.21,22 Finally, endometriosis tends to be associated with a negative impact on quality due to the symptoms of pelvic pain and infertility as well as from the effects of treatment.23 Given the nonspecific nature of many of these symptoms, endometriosis is often difficult to diagnose.
Findings on physical examination may include increased tenderness during a bimanual pelvic examination, a fixed retroverted uterus, lateral displacement of the cervix, decreased uterine mobility, uterosacral ligament nodularity, and enlarged adnexa (suggestive of an endometrioma). Most women with endometriosis will have a normal pelvic examination.24 There have been no laboratory findings strongly associated with endometriosis, although some have reported that increased CA-125 levels may be associated with severe disease; CA-125 is of limited value in women with minimal or mild disease.25
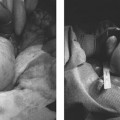
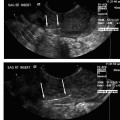
Imaging studies are of mixed value in diagnosing endometriosis, but are helpful in identifying associated findings that have endometriosis as an underlying etiology, including simple ovarian cysts, endometriomas, complex ovarian cysts, and ovarian torsion. Ultrasonography is commonly included in the evaluation of women with chronic pelvic pain and may identify adnexal findings consistent with an endometrioma. The appearance of endometriomas on ultrasound (preferably transvaginal) is described as homogeneous hypoechoic “tissue” of low level echoes, mostly multifocal, within the ovaries (Fig. 15.1).26 There should be absence of particular neoplastic features in these lesions.27 On color Doppler sonography (CDS), the lesions show poor or no vascularization.28 However, some reports indicate that CDS does not improve the diagnostic accuracy of transvaginal ultrasound alone in the diagnosis of endometriomas.29 Magnetic resonance imaging (MRI) is an excellent means for detection of macroscopic endometrial implants in the pelvis (particularly the posterior cul-de-sac and uterosacral ligaments) as areas of variable high signal intensity on T1-weighted images and low signal intensity on T2-weighted images.30 Other sequences, such as fat-suppressed imaging, are also useful for diagnosis (Fig. 15.2).
Although a presumptive clinical diagnosis is commonly assigned, the diagnosis is pathologic and typically confirmed with laparoscopy. Most physicians accept visualization of apparent lesions as enough to make a diagnosis of endometriosis.31 This approach, however, may lead to errors in diagnosis. Following a clinical diagnosis of endometriosis, laparoscopy has been found to confirm visible endometriosis in 70 to 90% of patients.12 However, the positive predictive value of visual findings compared with histologic findings varies from 14 to 65% based on the anatomic site of the lesion and from 0 to 76% based on the type of lesion present.32 For example, the positive predictive value is high in the posterior cul-de-sac, but low in unusual sites such as the psoas muscle. Overall, the positive predictive value for laparoscopic visualization is 43 to 45%.32,33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree