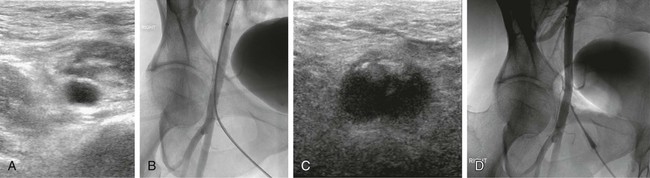
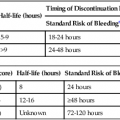
Manual compression (MC) after femoral arterial access removal has a high success rate with a relatively short compression time (5-15 minutes). The biggest drawback to MC is that 1 to 6 hours of postprocedure bedrest are required. The ideal duration of bedrest is unknown, but recent literature suggests that ambulation at 4 hours after MC for an 8F sheath and 3 hours after a 5F catheter placement may be safe.1,2 In patients with orthopnea, musculoskeletal disorders, or other conditions that make lying supine difficult or painful, it can be challenging or nearly impossible to manage the puncture site with MC. Anticoagulation and antiplatelet drugs can prolong the time to hemostasis and increase the risk of delayed complications with MC. Hematomas are the most common complication of MC, but most of these are self-limited.3 Other potential complications associated with MC include retroperitoneal hemorrhage, arteriovenous fistula (AVF), pseudoaneurysm, vessel thrombosis, and vasovagal reaction. Roughly 2% of MC leads to a major complication.4–7 The origins of the current interest in vascular closure devices is unclear but likely was the result of an effort to decrease the need for MC, speed the time to ambulation, and potentially reduce hematoma rates. Since the first edition of this chapter, their use has skyrocketed: in 2007 approximately 30% of the 10 million endovascular procedures performed in the United States employed a closure device.8 The most common indication for use of a closure device in our practice is a patient’s inability to comply with the bedrest required after MC. Examples include patients with chronic low back pain, severe chronic obstructive pulmonary disease, or nausea and vomiting. Prior to the availability of these closure devices, many of these patients would be denied angiography, require a femoral cutdown, or would be electively intubated for respiratory support or pain control. More clinically important is that these devices allow patients to have a procedure while on anticoagulation (thereby avoiding reversal to remove the femoral introducer) and allow patients to be started on anticoagulation or antiplatelet agents prior to completion of the procedure. This is associated with better outcomes, at least for coronary artery interventions. Closure devices are increasingly being used during aortic endograft or aortic valve prosthesis placement. In this application, very large caliber (up to 24F) puncture sites are preclosed using a suture-based device that allows the large arteriotomy to be drawn closed after the introducer sheath is removed.9,10 MC is difficult beyond a 10F sheath size but is also more challenging with larger (>7F) sheaths. Use of larger sheaths during interventional procedures is now common (e.g., covered stent placements for aneurysms, bare metal aortic stents for occlusive disease, balloon pumps for ventricular assistance, and the introducers required for endovascular treatment of patent ductus arteriosus and other large vessel embolizations). Not all the devices are approved for 8F sheaths, but there are studies demonstrating that some of these devices are reliable for closure of 8F sheaths.10 In the Equipment section, we will divide our discussion of aids to closure into hemostasis aids (Table e28-1), and invasive devices (Table e28-2). Aside from allergy, there are few contraindications to the noninvasive devices. Several contraindications are shared by the invasive devices: brachial artery closure due to possible nerve injury, a too-small vessel (<5 mm for most of the invasive devices), arteriotomy too large for chosen device, severely diseased vessels, systemic infection or infection in the region of the arteriotomy, an introducer sheath in place for a prolonged time (e.g., second day after a thrombolysis procedure), which may increase risk of infection, puncture into a bypass graft (relative contraindication), scarred groin that makes many of these devices more difficult to advance and deploy, and a known allergy to device components (bovine collagen, etc.). If repeat arterial access in the same groin is anticipated, devices that deposit a collagen plug outside the vessel are relatively contraindicated because of the induration they create over the femoral artery for several weeks (Fig. e28-1). Similarly, suture-based devices may create a fibrous cap that can complicate subsequent femoral cutdown for stent-graft placement.69 The use in antegrade access may also be a relative contraindication, although there are reports using the Perclose device,70 and there is no literature to suggest it imposes additional risks. TABLE e28-1 Commonly Available Aids to Hemostasis TABLE e28-2 *Published results or manufacturer recommendation for maximum access size closure. †Can close much larger arteriotomies by delivering sutures prior to dilatation of track.9 There are several classes of devices that can aid femoral artery closure (see Tables e28-1 and e28-2). The most mature class of devices is the group of compression devices that includes bedside C clamps and pneumatic compression straps.11–21,71 These do little to minimize groin complications or shorten bedrest time, but they do free up the operator and his/her assistants from compression. The second class is the topical agents; all the products in this class are procoagulants applied to the puncture site at the time of the MC. They are generally very safe, but their utility remains unproven because of the lack of appropriate clinical testing.22–34 It makes intuitive sense that these devices are most useful when the puncture is superficial, such as with radial artery punctures or dialysis graft access. The third class is invasive devices, but no foreign body is left behind. This class is represented by just two devices, an umbrella temporarily inserted into the artery and pulled back against the arteriotomy to provide temporary hemostasis, and a device that directs the arterial puncture to aid with hemostasis.35,36 The final class of devices are invasive devices, where the arteriotomy is mechanically or pharmacologically closed. This group includes all the devices that instantly seal the arteriotomy, but they are invasive in that they require the operator to deposit either a procoagulant (e.g., piece of bovine collagen) next to the artery or deliver a suture or clip into the arteriotomy.15,17,37–68 Each of these devices requires training and careful technique, introducing risks not usually associated with MC. First and foremost, exceptional sterile technique must be maintained throughout the procedure. Every patient must have a surgical-type skin preparation. Although controversial and not substantiated by experiment, antibiotic prophylaxis may be beneficial in preventing infection as well.72
Closure Devices
Clinical Relevance
Indications
Contraindications
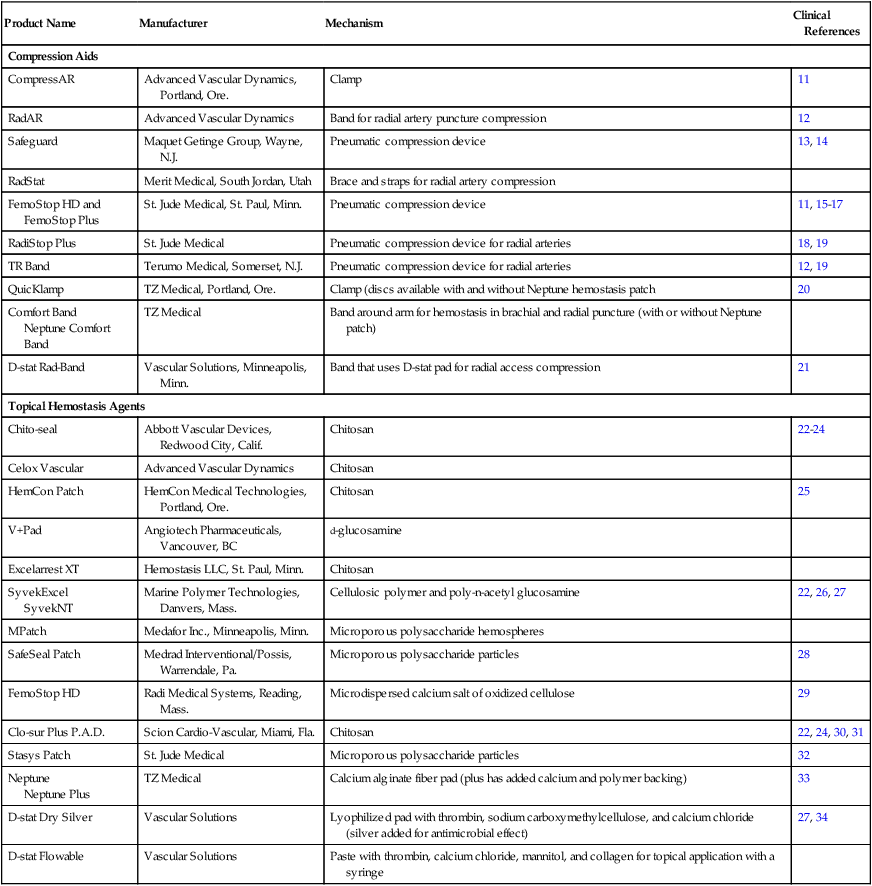
Product Name
Manufacturer
Mechanism
Clinical References
Compression Aids
CompressAR
Advanced Vascular Dynamics, Portland, Ore.
Clamp
11
RadAR
Advanced Vascular Dynamics
Band for radial artery puncture compression
12
Safeguard
Maquet Getinge Group, Wayne, N.J.
Pneumatic compression device
13, 14
RadStat
Merit Medical, South Jordan, Utah
Brace and straps for radial artery compression
FemoStop HD and FemoStop Plus
St. Jude Medical, St. Paul, Minn.
Pneumatic compression device
11, 15–17
RadiStop Plus
St. Jude Medical
Pneumatic compression device for radial arteries
18, 19
TR Band
Terumo Medical, Somerset, N.J.
Pneumatic compression device for radial arteries
12, 19
QuicKlamp
TZ Medical, Portland, Ore.
Clamp (discs available with and without Neptune hemostasis patch
20
Comfort Band
Neptune Comfort Band
TZ Medical
Band around arm for hemostasis in brachial and radial puncture (with or without Neptune patch)
D-stat Rad-Band
Vascular Solutions, Minneapolis, Minn.
Band that uses D-stat pad for radial access compression
21
Topical Hemostasis Agents
Chito-seal
Abbott Vascular Devices, Redwood City, Calif.
Chitosan
22–24
Celox Vascular
Advanced Vascular Dynamics
Chitosan
HemCon Patch
HemCon Medical Technologies, Portland, Ore.
Chitosan
25
V+Pad
Angiotech Pharmaceuticals, Vancouver, BC
d-glucosamine
Excelarrest XT
Hemostasis LLC, St. Paul, Minn.
Chitosan
SyvekExcel
SyvekNT
Marine Polymer Technologies, Danvers, Mass.
Cellulosic polymer and poly-n-acetyl glucosamine
22, 26, 27
MPatch
Medafor Inc., Minneapolis, Minn.
Microporous polysaccharide hemospheres
SafeSeal Patch
Medrad Interventional/Possis, Warrendale, Pa.
Microporous polysaccharide particles
28
FemoStop HD
Radi Medical Systems, Reading, Mass.
Microdispersed calcium salt of oxidized cellulose
29
Clo-sur Plus P.A.D.
Scion Cardio-Vascular, Miami, Fla.
Chitosan
22, 24, 30, 31
Stasys Patch
St. Jude Medical
Microporous polysaccharide particles
32
Neptune
Neptune Plus
TZ Medical
Calcium alginate fiber pad (plus has added calcium and polymer backing)
33
D-stat Dry Silver
Vascular Solutions
Lyophilized pad with thrombin, sodium carboxymethylcellulose, and calcium chloride (silver added for antimicrobial effect)
27, 34
D-stat Flowable
Vascular Solutions
Paste with thrombin, calcium chloride, mannitol, and collagen for topical application with a syringe
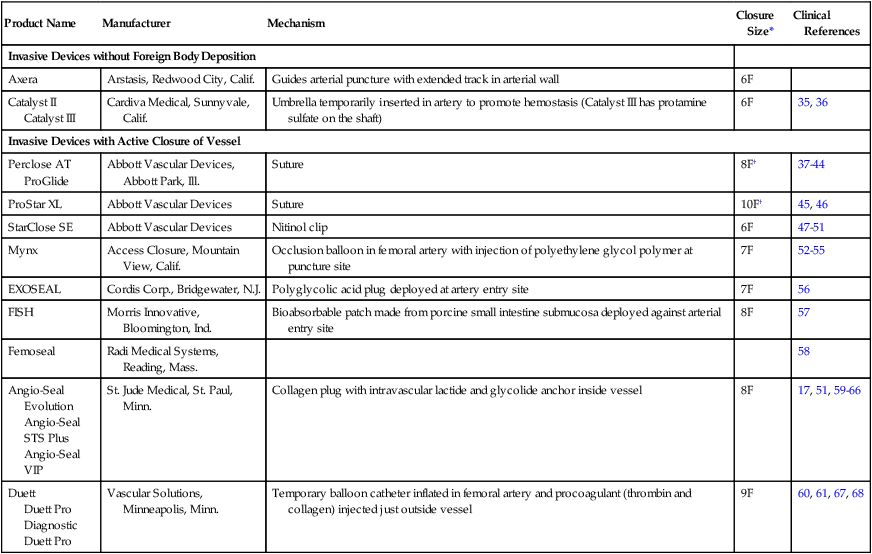
Product Name
Manufacturer
Mechanism
Closure Size*
Clinical References
Invasive Devices without Foreign Body Deposition
Axera
Arstasis, Redwood City, Calif.
Guides arterial puncture with extended track in arterial wall
6F
Catalyst II
Catalyst III
Cardiva Medical, Sunnyvale, Calif.
Umbrella temporarily inserted in artery to promote hemostasis (Catalyst III has protamine sulfate on the shaft)
6F
35, 36
Invasive Devices with Active Closure of Vessel
Perclose AT ProGlide
Abbott Vascular Devices, Abbott Park, Ill.
Suture
8F†
37–44
ProStar XL
Abbott Vascular Devices
Suture
10F†
45, 46
StarClose SE
Abbott Vascular Devices
Nitinol clip
6F
47–51
Mynx
Access Closure, Mountain View, Calif.
Occlusion balloon in femoral artery with injection of polyethylene glycol polymer at puncture site
7F
52–55
EXOSEAL
Cordis Corp., Bridgewater, N.J.
Polyglycolic acid plug deployed at artery entry site
7F
56
FISH
Morris Innovative, Bloomington, Ind.
Bioabsorbable patch made from porcine small intestine submucosa deployed against arterial entry site
8F
57
Femoseal
Radi Medical Systems, Reading, Mass.
58
Angio-Seal Evolution
Angio-Seal STS Plus
Angio-Seal VIP
St. Jude Medical, St. Paul, Minn.
Collagen plug with intravascular lactide and glycolide anchor inside vessel
8F
17, 51, 59–66
Duett
Duett Pro
Diagnostic Duett Pro
Vascular Solutions, Minneapolis, Minn.
Temporary balloon catheter inflated in femoral artery and procoagulant (thrombin and collagen) injected just outside vessel
9F
60, 61, 67, 68
Equipment
Technique
Technical Aspects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Closure Devices