Anatomy
- ◼
Adenomas can develop anywhere in the colon or rectum but are seen with the greatest frequency in the sigmoid colon.
- ◼
The frequency of polyp occurrence in each section of the colon is approximately as follows: rectum 15%; sigmoid colon 25%; descending colon 10%; transverse colon 20%; ascending colon 20%; and cecum 10%.
Pathophysiology
- ◼
Colon cancer is the third most commonly diagnosed cancer and third most common cause of cancer-related death in both men and women.
- ◼
The lifetime risk for developing colorectal carcinoma is approximately 5%.
- ◼
It is well established that there is a reduction in colorectal carcinoma incidence after colonoscopic polypectomy, indicating that screening techniques are essential in the prevention and early detection of colorectal carcinoma.
- ◼
Colonic polyps are growths into the bowel lumen that can develop as isolated polyps or in the setting of polyposis syndromes.
- ◼
Isolated colonic polyps of all sizes are seen in approximately 37.6% of the screening population, whereas potentially clinically significant polyps 6 mm or greater have a prevalence of 14%.
- ◼
Polyps are histologically characterized as adenomatous, hyperplastic, and other. The “other” category includes juvenile/hamartomatous polyps, inflammatory polyps, lymphoid aggregates, mucosal tags, and submucosal lipomas.
- ◼
Only adenomatous polyps are of concern with respect to colon cancer.
- ◼
The adenoma is a precursor lesion that can potentially harbor dysplasia and develop into colon cancer; this prevailing view on the pathogenesis of colon cancer is called the adenoma-carcinoma sequence.
- ◼
Adenomas are further characterized into three subtypes based on their histological architecture: tubular, tubulovillous, and villous. Adenomas containing less than 25% villous features are classified as tubular adenomas; those with 25% to 75% villous features are tubulovillous adenomas; those with more than 75% villous features are villous adenomas.
- ◼
Adenomas also can be characterized based on the degree of cellular atypia seen on pathology (mild, moderate, or severe dysplasia), depending on the amount of nuclear changes and number of mitotic figures.
- ◼
With imaging studies and/or colonoscopy as screening tools, the radiologist and gastroenterologist cannot visually distinguish among the different types of polyps, nor can they determine the histological factors or degree of cellular atypia of an adenoma. Thus, generally all encountered polyps seen on colonoscopy and those meeting a certain size threshold on noninvasive studies, such as computed tomography colonography (CTC) are removed.
Colon cancer screening
- ◼
The goal of any cancer screening, including colon cancer screening, is to achieve a reduction in mortality by identifying disease at earlier stages and thus reducing the incidence of advanced disease.
- ◼
Screening studies should be targeted toward the removal of adenomas that have the highest potential for developing into colorectal carcinoma.
- ◼
These “advanced adenomas” have traditionally been defined by any of the following three criteria: high-grade dysplasia, size 1 cm or greater, or a substantial (>25%) villous component (i.e., tubulovillous or villous adenomas).
- ◼
In an asymptomatic screening population, the overall prevalence of an advanced adenoma or carcinoma is 3%.
- ◼
The 5-year survival is 90% for disease confined to the wall of the bowel; however, it falls to 68% for regional disease and plummets to 10% for metastatic disease.
- ◼
The American Cancer Society first issued formal guidelines for colorectal screening in 1980, followed by guidelines from the U.S. Preventive Services Task Force, the American College of Radiology, and the U.S. Multi-Society Task Force on Colorectal Cancer. Although there are differences among the guidelines, there is growing consensus, with the latest iteration being a collaborative effort by all of these groups.
- ◼
The recommendations include continued support of the fecal occult blood test (FOBT), as well as the fecal immunohistochemical test and stool deoxyribonucleic acid (DNA) testing, assuming a patient is willing to undergo an invasive procedure in the instance of a positive test. The recommendations prefer tests that detect lesions earlier, including optical colonoscopy (OC) every 10 years or flexible sigmoidoscopy (FSIG), CTC, or double-contrast barium enema every 5 years.
Screening modalities
Fecal occult blood test and other stool-based examinations
- ◼
The principle behind the FOBT and other stool-based examinations is that invasive carcinoma will cause bleeding, which can be detected using methods, such as guaiac staining, immunohistochemistry, and DNA.
- ◼
Small polyps tend not to bleed, and larger lesions tend to bleed intermittently. As a result, these tests are subject to sampling error and are more likely to detect cancer rather than precancerous lesions.
- ◼
FOBT is supported by randomized controlled studies, which demonstrate that its use leads to cancers being detected at an earlier and more curable stage compared with no screening, leading to reductions in colorectal cancer mortality of 15% to 33%.
- ◼
The limitations of stool testing include the necessity for annual testing, low individual test sensitivity, and the need to follow any positive test with an invasive test. Furthermore, the sensitivity is highly variable and depends on hydration status of the feces, brand and style of the test, as well as chosen target lesion (i.e., adenoma vs. carcinoma).
Flexible sigmoidoscopy
- ◼
FSIG is an optical endoscopic procedure that examines the most distal portion of the colon lumen.
- ◼
FSIG is associated with a 60% to 80% reduction in colorectal cancer mortality for the area of colon within its reach. Overall decreased incidence compared with an unscreened group also has been demonstrated. Its principal advantages over colonoscopy are that it requires a less extensive bowel preparation than a full colonic examination, and because it is less invasive than colonoscopy, sedation is not required. It also allows for concurrent sampling and/or removal of detected lesions.
- ◼
Like colonoscopy, FSIG can be complicated by patient discomfort and, more importantly, bowel perforation. Its greatest limitation, however, is its failure to evaluate the entire colon.
- ◼
The U.S. Multi-Society Task Force considers that either 5- or 10-year intervals are acceptable but favors 10-year intervals.
Colonoscopy
- ◼
Colonoscopy is an optical procedure that evaluates the entire colon. It is one of the most commonly performed medical procedures. Patients require an extensive colonic cleansing preparation, and the procedure is performed under sedation. Because it allows for direct optical visualization, biopsies are often performed.
- ◼
The greatest advantage to colonoscopy is that usually the entire colon can be screened and suspicious lesions sampled in a single visit. The seminal paper by Winawer and colleagues demonstrated a reduction in incidence of colon cancer of 76% to 90%.
- ◼
Although no prospective randomized controlled trial has been performed to evaluate colonoscopy, its health benefits are undisputed. A U.S. Department of Veterans Affairs study demonstrated a 50% reduction in mortality when colonoscopy was performed on a symptomatic population.
- ◼
The limitations of colonoscopy include the need for colon cleansing and patient sedation. Because of sedation, a patient chaperone is needed. Controlled studies have shown the colonoscopy miss rate for adenomas 10 mm or larger to be 6% to 12%.
- ◼
The additional risks of colonoscopy related to sedation and biopsy include cardiopulmonary events, such as arrhythmia and hypotension, as well as postpolypectomy bleeding and perforation.
- ◼
Colonoscopy every 10 years beginning at age 50 years (45 years in African Americans) is recommended as a screening option for colorectal cancer.
Computed tomography colonography
- ◼
CTC is a minimally invasive examination that uses CT to acquire images of the colon and two-dimensional (2D) and three-dimensional (3D) displays for interpretation. The mechanism and technique are explained in detail later in this chapter.
- ◼
CTC evaluates the entire colon, including those segments that are particularly redundant and difficult to evaluate with colonoscopy. In addition, the examination can be performed without sedation. The examination can also detect extracolonic findings. In one study, 9% of the total patients had clinically important extracolonic findings. Metaanalysis of 33 studies on nearly 6400 patients demonstrated an 85% to 93% sensitivity and 97% specificity for polyps 10 mm and larger. The 96% sensitivity of CTC for invasive cancer was comparable to that of OC.
- ◼
One of the greatest limitations of CTC is its availability. Because funding is not widely available for screening CTC, the professional capacity is markedly limited.
- ◼
CTC requires a cathartic bowel preparation, although studies are underway to eliminate this need. The most notable limitation is that CTC is an imaging study only, and patients must be referred to OC for biopsy and/or removal if a significant colonic lesion is encountered.
- ◼
CTC every 5 years for patients older than 50 years of age (45 years in African Americans) is an acceptable screening option for colorectal cancer.
Techniques
Computed Tomography Colonography (CTC) can be entirely performed by a technologist after adequate training with minimal assistance from a radiologist. However, the presence of an onsite radiologist is preferred for consultation in difficult cases. Successful CTC examination involves the following:
- 1.
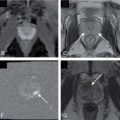
Colonic cleansing with tagging of residual stool and luminal fluid ( Fig. 9.1 and Table 9.1 ).

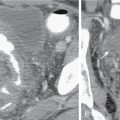
Fig. 9.1
Intraluminal fluid ( arrows , axial computed tomography [CT] image in A and three-dimensional volume rendered image in B) may limit interpretation of CT colonography.
Table 9.1
Computed Tomography Colonography Bowel Preparation Protocols
Preparation
Day Before Examination
Morning of Examination
Additional Instructions
Sodium phosphate
6 PM: 45 mL of sodium phosphate diluted in 4 oz of water orally
9 PM: Four bisacodyl tablets (5-mg each) orally
10-mg bisacodyl suppository approximately 1 hour before the examination
Refrain from eating solids and maintain adequate hydration with clear liquids
Magnesium citrate
4 PM: 200–300 mL (10 oz) of magnesium citrate orally
6 PM: Four bisacodyl tablets are taken orally with 8 oz of water
10-mg bisacodyl suppository approximately 1 hour before the examination
- 2.
Colonic distention.
- 3.
Data acquisition.
- 4.
Visualization of CTC with 2D and 3D techniques.
Bowel cleansing
- ◼
Patient is instructed to drink clear liquids the day before CTC.
- ◼
The main bowel-cleansing agents include cathartics, such as magnesium citrate and sodium phosphate and gut lavage solutions, such as polyethylene glycol (PEG).
- ◼
Sodium phosphate is an oral saline cathartic and is referred to as a “dry prep” because it leaves behind little residual fluid in the colon.
- ◼
Magnesium citrate is also a saline cathartic that causes fluid to accumulate in the bowel because of its osmotic effects and promotes peristaltic activity and bowel emptying.
- ◼
PEG is an effective agent for cleansing the bowel, but results in excessive fluid retention in the colon. It is considered a “wet prep” and is not ideal for virtual colonoscopy.
- ◼
Dual-positive oral contrast agents are used for tagging of the residual stool and luminal fluid after catharsis. Barium and/or iodine solutions are ingested with meals, usually in conjunction with the oral cathartics, 24 to 48 hours before imaging to allow adequate incorporation of the positive contrast material with colonic contents.
- ◼
Tagged stool and residual fluid demonstrate higher attenuation and are easily discernible from the homogeneous soft tissue density of polyps and colonic folds.
Colonic distention
Room air
- ◼
Advantages of room air include ease of use, ready availability, and lack of additional cost. It provides good colonic distention.
- ◼
However, because room air is composed predominantly of nitrogen, it is poorly absorbed through the colonic wall and patients can experience abdominal discomfort and pain after CTC until the air is expelled distally by peristalsis.
- ◼
With the patient in the left lateral decubitus (LLD) position, a small rubber catheter is used to insufflate the colon with a hand-held bulb syringe. Using an insufflation bulb, up to 70 puffs or 2 L of room air is administered until the patient experiences fullness or mild discomfort, which signals that the colon is well distended.
Carbon dioxide
- ◼
Preferred method per the American College of Radiology.
- ◼
A commercially available electronic insufflation device provides a constant flow of CO 2 into the colon per rectum at a relatively low level of preset pressure to reduce the risk for colonic perforation.
- ◼
CO 2 is absorbed rapidly through the colonic wall and exhaled through the lungs.
- ◼
There is decreased postprocedural discomfort and improved colonic distention using automated CO 2 compared with patient-controlled room air.
- ◼
In the automated CO 2 technique, a small-caliber, flexible rectal catheter is placed with the patient in the LLD position, and 1.0 to 1.5 L CO 2 is delivered by the automated device at an equilibrium pressure of approximately 20 mm Hg.
- ◼
The patient is moved into the right lateral decubitus position until approximately 2.5 L of CO 2 has been introduced.
- ◼
The total volume of CO 2 used in the procedure can vary from 2 to 10 L owing to individual differences in colonic volume, colonic resorption, and reflux through the ileocecal valve ( Fig. 9.2 ).