Mitral regurgitation
Occurs in 60–90 % of patients with aortic stenosis
Generally reduced after aortic valve replacement
Early data indicate no increased mortality in patients with versus without mitral regurgitation after TAVR
TAVR may potentially worsen mitral regurgitation by restriction of anterior leaflet mobility
Coronary artery disease s/p myocardial infarction
No excess risk factor association with prevalence of aortic stenosis when comparing AS patients with controls
Discordant data as to prognosis in patients with versus without coronary artery disease following TAVR
Asymmetric septal hypertrophy
Severe asymmetric hypertrophy may onsider septal myomectomy
Cotraindication to TAVR
Mitral Regurgitation
Mitral regurgitation (MR) can result from structural abnormalities of the mitral valve apparatus—including leaflets, annulus, chordae, and papillary muscles—or it may ensue from mechanisms such as dyssynchronous contraction. Ischemic heart disease may cause MR via annular dilatation, papillary muscle infarction or ischemia, or systolic tenting of the valve leaflet due to adverse LV remodeling following infarction (Figs. 17.1, 17.2, and 17.3). Often, multiple mechanisms coexist, just as nonischemic cardiomyopathy may be superimposed on ischemic heart disease and explain LV enlargement or dysfunction out of proportion to the extent of coronary artery disease.
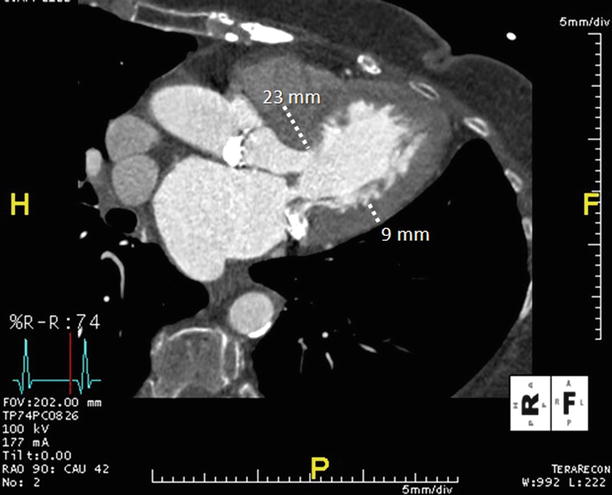
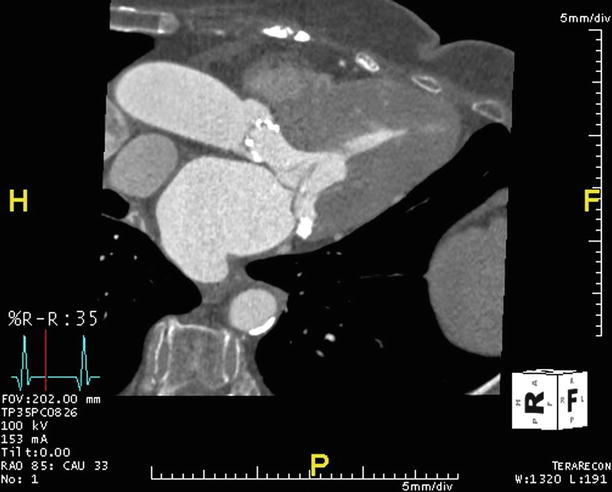
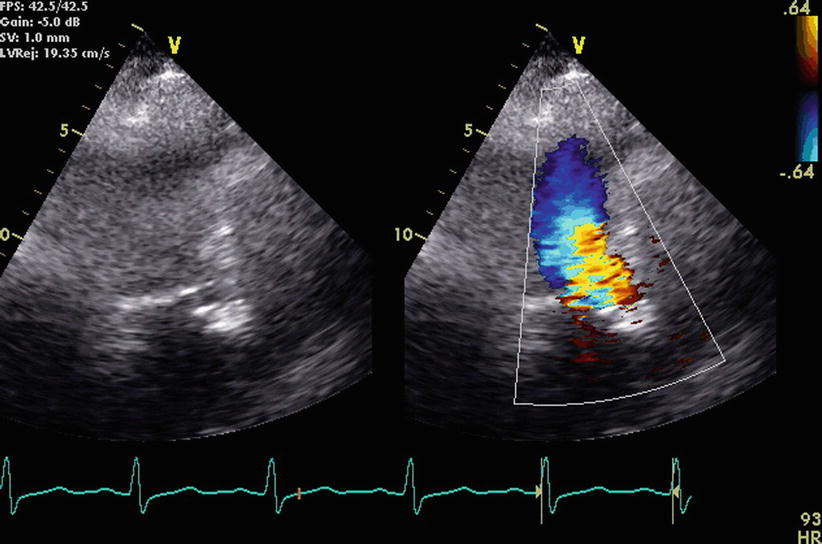

Fig. 17.1
Asymmetric septal hypertrophy is evident in this ECG-gated, multiplanar reformatted CTA that has been reconstructed in the three-chamber or LV outflow tract plane. This end-diastolic reconstruction allows measurement of the anteroseptum (23 mm) and inferolateral (9 mm) LV myocardial segments, with marked asymmetric thickening of the septum

Fig. 17.2
The same CTA data acquired for Fig. 17.1 is now reconstructed at an end-systolic phase, revealing systolic encroachment into the left ventricular outflow tract by the asymmetrically thickened septum

Fig. 17.3
In the same patient with evident ASH by CTA shown in Figs. 17.1 and 17.2, echocardiography acquired during systole (left) and with superimposed Doppler recordings (right) is shown from the apical 3-chamber view, indicating flow acceleration and mosaic colors with aliased velocities consistent with some degree of left ventricular outflow tract obstruction
Some degree of mitral regurgitation coexists in 60–90 % of patients with AS [3–5]. When overlaid on a condition that fundamentally limits cardiac output, MR further detracts from forward stroke volume necessary for perfusion, exacerbates left atrial and pulmonary venous hypertension, and increases the likelihood of atrial fibrillation. Increased LV pressures in AS, in turn, may exacerbate MR. “Functional” MR is defined as MR in the absence of structural abnormalities of the mitral apparatus and contributes to excess mortality post-AVR. Jeong et al. observed a hazard for late cardiac death of more than fivefold in patients with mild to moderate functional MR in patients undergoing AVR when compared to patient with no to trivial MR (p = 0.016) [4]. In a retrospective study of elderly patients who had undergone surgical AVR, moderate MR was also shown to be an independent predictor of late mortality [6].
The underlying processes associated with the progression of calcific aortic stenosis commonly affects the mitral valve as well; prior rheumatic injury may further contribute to structural alterations of the mitral apparatus. In advanced disease, it may be difficult (and unnecessary) to ascertain the underlying cause of MR; nonetheless, its presence and severity are generally evident by routinely transthoracic echocardiography (Figs. 17.4 and 17.5).
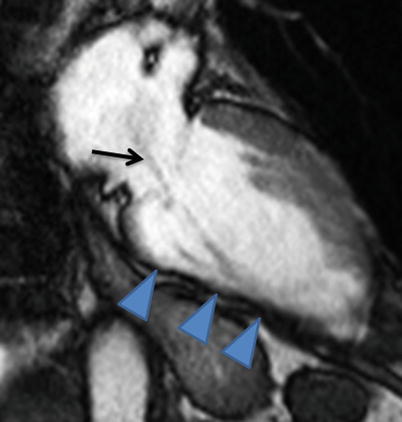
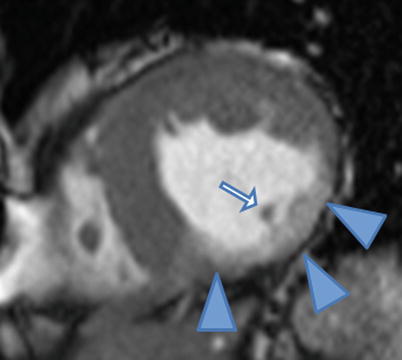

Fig. 17.4
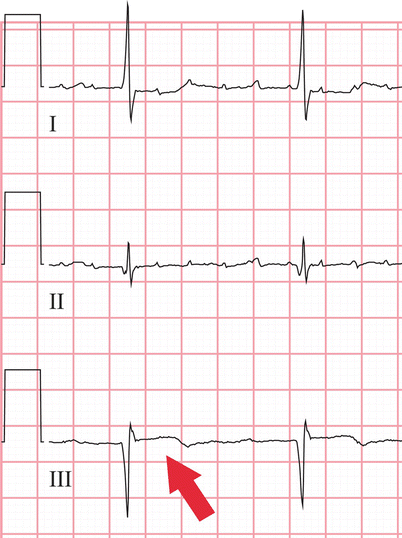
Systolic frame from a vertical long-axis cine cardiac magnetic resonance acquisition shows a thinned inferior wall (arrowheads) due to prior myocardial infarction as well as a jet of turbulent mitral regurgitation flow (arrow)

Fig. 17.5
Inferior wall thinning and contrast enhancement (arrowheads) are evident on this end-diastolic frame from a mid-level short-axis cine cardiac magnetic resonance acquired after contrast injection. Also note the relatively reduced mass of the posteromedial papillary muscle (arrow) compared to the anterolateral papillary muscle
When severe MR is present in patients with severe AS requiring surgery, addressing both valves (e.g., AVR plus mitral valve repair) is advocated. However, for moderate MR, concomitant mitral valve repair (MVR) with AVR is a subject of debate [4]. Surgical replacement of both valves results in a higher operative risk [7, 8], and some studies suggest that moderate functional MR may regress after isolated AVR through reverse LV remodeling [9, 10]. Tassan et al. found that MR severity decreased early after AVR in a cohort of patients with normal LVEF, moderate MR, and severe aortic stenosis [11]. Lower pulmonary artery pressure and reduction in mitral annular dimension and LV mass predicted regression of MR in this echocardiography-guided investigation [11]. However, moderate to severe MR may not improve and may even increase post-AVR [10, 12, 13]. Thus, some argue for an aggressive approach for concomitant MR and AS. Vanden Eynden et al. found improved postoperative MR predicted better 10-year survival post-AVR, noting little improvement in those whose MR was a result of rheumatic disease or myxomatous degeneration [14]. While data suggest that patients with uncorrected MR after AVR have higher morbidity and mortality, ongoing evidence must accrue to ensure that correcting mild to moderate MR with aortic valve replacement consistently confers additional postoperative improvements over AVR alone [4].
In the TAVR era, studies are appropriately focused on the outcomes of patients with MR who undergo transcatheter heart valve implantations. A prospective, single-center study by D’Onofrio et al. performed echocardiography in 176 patients undergoing TAVR prior to and at discharge, 1, 3, 6, and 12 months post-procedure [15]. Despite a trend toward higher in-hospital mortality after TAVR in patients with MR as compared to patients with no MR, 20-month survival was identical in those with moderate or more severe MR compared to those with mild or less MR. In addition, MR severity fell and LV remodeling improved after TAVR.
Other studies support variable improvement of functional MR after TAVR. Durst et al. reviewed echocardiograms before and at 1-day, 3-month, and 6-month post-TAVR in 35 patients enrolled in the REVIVAL II trial (tRanscatheter EndoVascular Implantation of VALves) [16]. Those with at least 30 % reduction in vena contracta-based MR severity comprised approximately one-third of the group—so-called good responders—while the rest were deemed “poor responders.” Poor responders were distinguished from good responders by the presence of mitral annular calcification causing restriction of mitral leaflet motion. Hekimian et al. studied MR in a prospective study of 119 patients that included baseline, 7-day, and 1-month post-TAVR echocardiograms [5]. Improvement in both organic and structural MR was seen at 7 days, was not influenced by the overlap of the anterior mitral leaflet and the device, and was associated with improvement in LV ejection fraction. Others have concluded that even if there is no improvement in the degree of MR after TAVR, the correction in aortic valve pathology is sufficient to improve patient outcomes by reducing left ventricular dimensions [15]. These results call for more studies with longer-term follow-up to evaluate outcomes and survival after TAVR in patients with MR.
Technical aspects of TAVR in patients with coexistent MR have been considered. Cilingiroglu and Hakeem have commented on the variable results from studies evaluating the impact of TAVR with CoreValve or Edwards Sapien valve on mitral valve function [17]. The CoreValve prosthesis can potentially worsen mitral regurgitation due to mitral annular calcification because it is usually placed 8 mm below the base of the aortic root and can potentially restrict anterior leaflet mobility [17–19]. Reproducible measurements are necessary to quantify MR as part of the careful pre-TAVR evaluation, and this information should aid in the selection of patients likely to benefit from TAVR and to guide approach, type of valve, and device position.
As with patient selection for TAVR, transcatheter mitral valve repair may be a consideration for the poor surgical candidate with significant MR. Rudolph et al. reported findings in 11 patients who underwent MitraClip placement as well as TAVR [20]. Despite technical feasibility, clinical benefit was limited, underscoring the need for further investigations as to the implications of MR on TAVR and additional interventions in the setting of significant AS.
Prior Myocardial Infarction
Aortic stenosis and coronary artery disease (CAD) often coexist. Age-related calcified aortic sclerosis has been associated with a 50 % increased risk of cardiovascular mortality and myocardial infarction in a longitudinal study of over 5,000 patients [21]. The Cardiovascular Health Study studied clinical factors associated with aortic sclerosis and stenosis in a population-based evaluation that included echocardiography [22]. Independent predictors of degenerative aortic valve disease included age, male gender, smoking, and hypertension—all risk factors for atherosclerosis. Furthermore, high lipoprotein(A) and low-density lipoprotein cholesterol levels contributed to degenerative aortic valve disease risk [22]. In contrast, Ortlepp et al. observed no excess risk factor association with prevalence of aortic stenosis when comparing AS patients with controls matched for age, sex, and angiographically defined coronary artery disease [23]. Indirectly supportive of these findings, a recent meta-analysis of statin therapy trials for aortic valve disease confirmed that treating hyperlipidemia, an established approach to attenuate atherosclerosis, does not halt progression of AS [24]. These data suggest that AS and CAD, while potentially occurring amidst a backdrop of similar risk factors, may develop via distinct mechanistic pathways. Given the excess prevalence of coronary atherosclerosis in patients referred for TAVR (Figs. 17.6, 17.7 and 17.8), thorough evaluation of CAD in this population should and is nevertheless the standard of care.
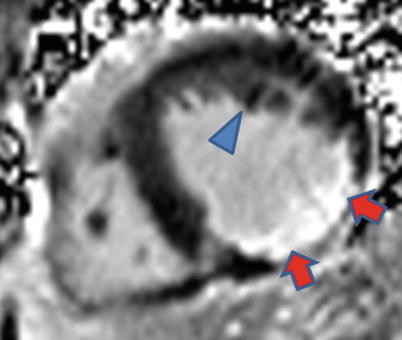
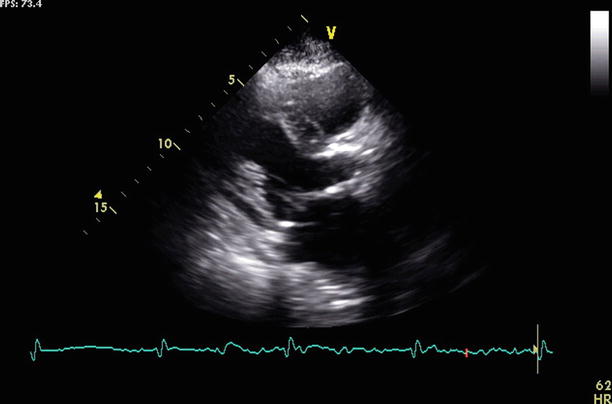
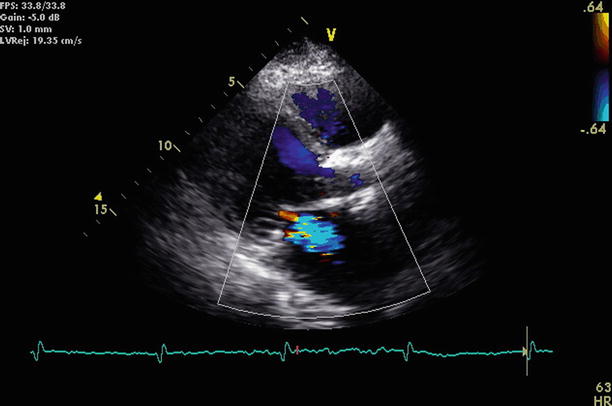

Fig. 17.6
Late post-gadolinium enhancement cardiac magnetic resonance imaging obtained 10 min after contrast shows extensive inferior wall infarct scar (arrows). Not seen is the posteromedial papillary muscle, which should be dark like the anterolateral papillary muscle (arrowhead)

Fig. 17.7
Still frame from a transthoracic echocardiogram, parasternal view shows mitral annular calcification in a pre-TAVR patient

Fig. 17.8
Still frame from the same parasternal view shown in Fig. 17.7 with superimposed color Doppler demonstrates a jet of mitral regurgitation
Previous studies have demonstrated that for surgical AVR, history of CAD and/or previous CABG is an independent risk factor for increased mortality [25–27]. Dewey et al. showed that CAD, as defined by previous percutaneous intervention (PCI) or coronary artery bypass graft (CABG), is a significant risk factor for mortality in patients undergoing TAVR [28]. In that study, data on operational and long-term survival was collected on 201 patients undergoing TAVR. Concomitant CAD was identified by previous cardiovascular intervention. Patients who had CAD were tenfold more likely to die within 30 days of the procedures than those without CAD. However, it is unrealistic to exclude CAD or prior MI from TAVR candidacy.
Webb et al. study reported functional improvement after TAVR in a population where 73 % had a history of previous myocardial infarction, a finding that was not associated with increased mortality at up to 2 years [29]. Drews et al. reported technical success and subsequent survival post-TAVR were similar in patients with versus without a history of previous heart surgery [30]. Ducrocq et al. also evaluated the procedural impact of previous coronary bypass surgery [31]. The study found similar 30-day mortality post-TAVR in patients with versus those without a history of CABG. Collectively, these data further position TAVR as an attractive option to repeat sternotomy or thoracotomy in patients with CAD that previously required cardiac surgery.
Elective TAVR may be deferred in the setting of recent myocardial infarction (MI) (Fig. 17.9). With high prevalence of anatomic CAD detected by angiography in patients with symptomatic AS, patients who are considered too high risk for valve surgery may require catheter-based approaches to treat both CAD and AS. Pasic et al. reported initial results of combining percutaneous coronary intervention with transapical TAVR [32]. They reported technical feasibility in 46 patients undergoing both procedures simultaneously and 30-day mortality similar to what has been reported with TAVR alone. Larger randomized trials are needed to know if elective PCI combined with TAVR delivers the posited benefit of reducing post-TAVR myocardial infarction without increasing procedural complication rates. Such data may help formulate guidelines regarding when and how obstructive CAD should be treated in patients referred for TAVR.
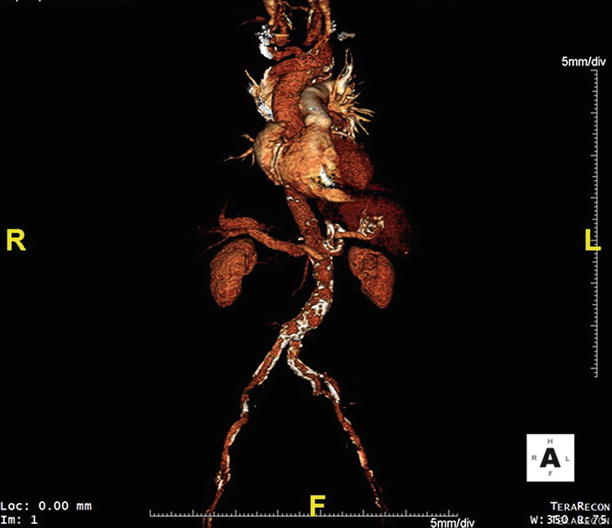

Fig. 17.9
Extensive abdominal aortic atherosclerosis is seen in this anteroposterior volume rendering of CTA data obtained during pre-TAVR evaluation
Asymmetric Septal Hypertrophy
Asymmetric septal hypertrophy (ASH) implies nonuniform thickening of the interventricular septum in relation to the remaining myocardial segments [33, 34]. Left ventricular hypertrophy (LVH) is most commonly a compensatory mechanism for ventricular pressure overload, either due to a rise in circulatory pressure or by a mechanical obstruction to outflow [35]. As significant aortic valve stenosis is the sine qua non prompting TAVR consideration, some degree of LVH or concentric thickening relative to a small LV cavity size is almost uniformly present in patients being evaluated for TAVR (Figs. 17.10 and 17.11).
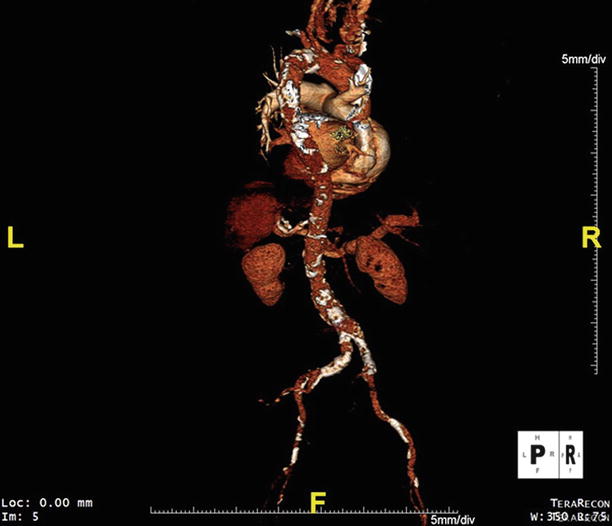
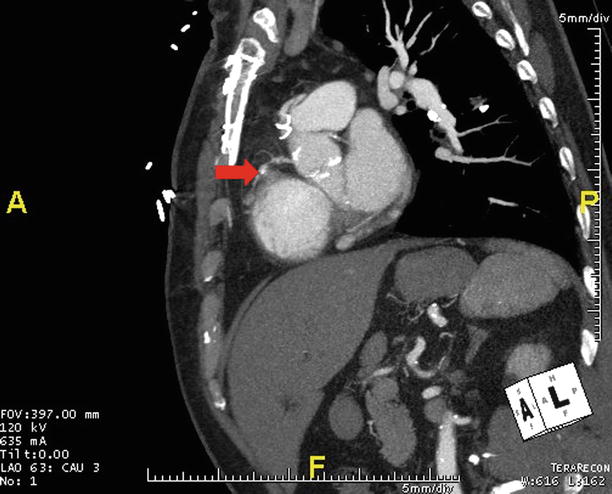

Fig. 17.10
This posteroanterior volume rendering of CTA data obtained during pre-TAVR evaluation shows atherosclerosis in the thoracic and abdominal aorta

Fig. 17.11
CTA obtained prior to TAVR is shown in an oblique sagittal maximum intensity projection in a patient with prior myocardial infarction due to right coronary artery occlusion (arrow)
The histopathology of LVH that develops in response to aortic stenosis involves muscle fiber hypertrophy and abnormalities of the collagen network [33, 36, 37]. Hypertrophy can be uniform and symmetric or it is most pronounced in the ventricular septum leading to ASH. ASH may lead to forward motion of the anterior leaflet of the mitral valve during systole (SAM), resulting in mitral insufficiency. ASH may also be associated with dynamic left ventricular outflow tract obstruction (LVOTO, Fig. 17.12). While ASH may conjure a possible diagnosis of hypertrophic cardiomyopathy (HCM), it should be noted that patients with HCM can coincidentally develop intrinsic disease of the aortic valve [38]. Asymmetric thickening may commonly be present in hypertension that is, in turn, a common comorbid condition in the pre-TAVR patient [39, 40]. Its presence in hypertensive patients has been shown to confer increased cardiovascular risk [40, 41].