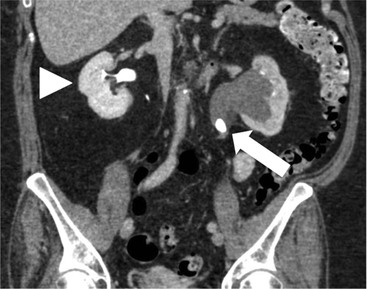
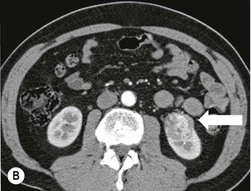
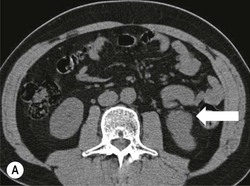
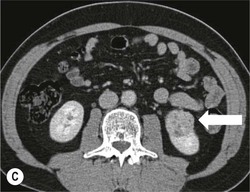
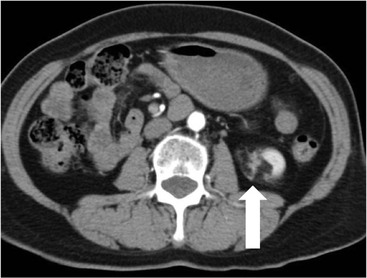
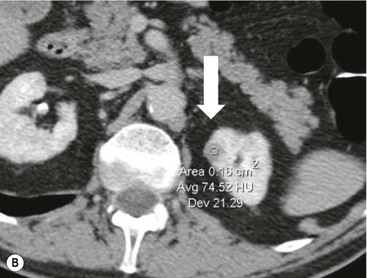
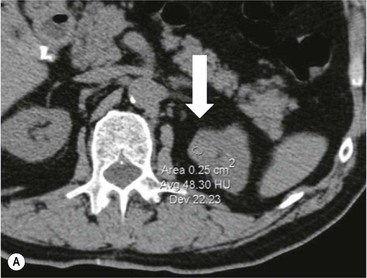
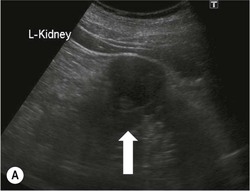
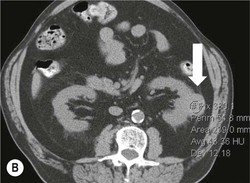
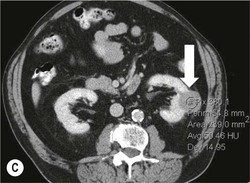
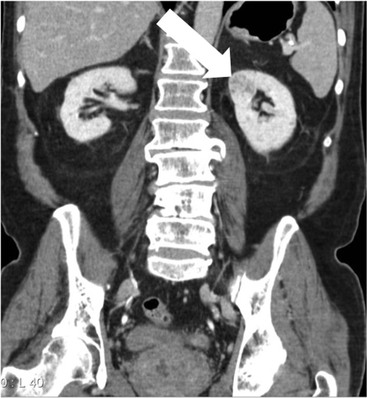
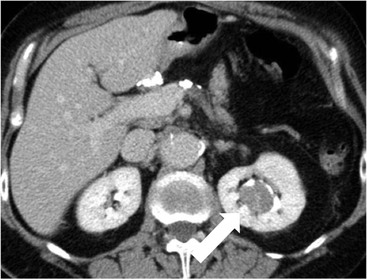
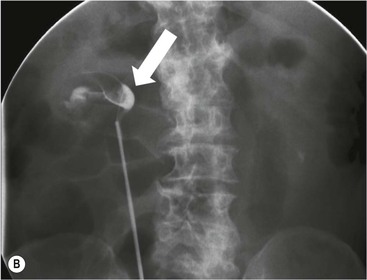
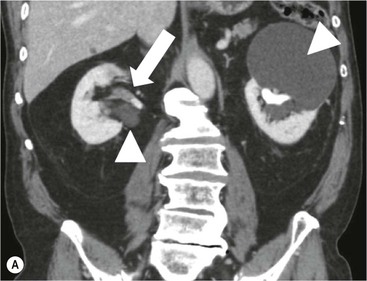
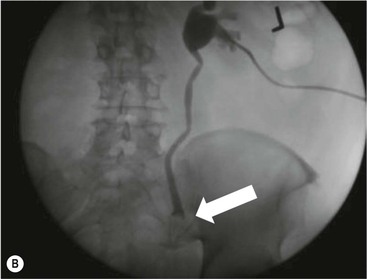
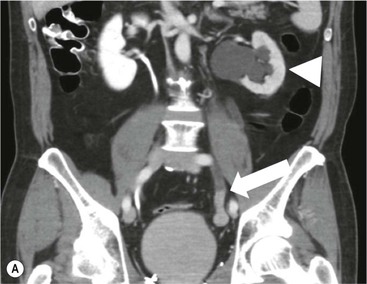
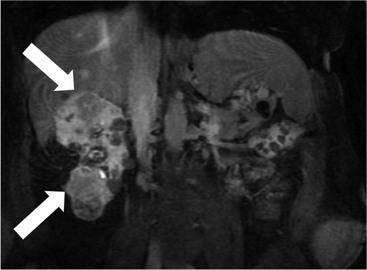
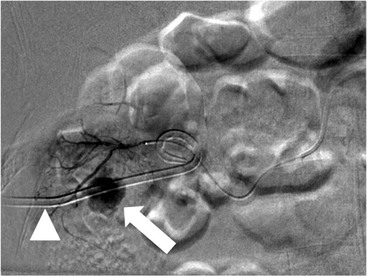
Owen J. O’Connor, Tarek El-Diasty, Mohamed Abou El-Ghar, Michael M. Maher Haematuria refers to the presence of red blood cells in urine. Radiological referral of selected patients is often necessary as part of clinical management for this problem; however, the optimal method of patient selection and the best imaging algorithm are subject to debate.1 In this chapter we will outline current opinion regarding best practices for such patients. Haematuria visible to the naked eye is said to be macroscopic, but when a microscope is required for detection, the term microscopic haematuria is used. Macroscopic haematuria is a strong predictor of serious underlying urological pathology. In one large study, 21% of patients with macroscopic haematuria were found to have malignancy, and repeat assessment yielded malignant findings in 12 of 35 (17%) patients with persistent symptoms, though initial tests were negative.2 Microscopic haematuria is often initially detected using a urine dipstick test: a simple inexpensive examination that is useful if positive but insufficient to exclude haematuria on its own, especially at a single time point. Screening for haematuria in asymptomatic patients is of dubious value, since less than 2% of young patients with a positive dipstick test have a serious treatable urinary tract disease and data are conflicting in older patients.3 In one study, 75% of patients referred for consultation following a positive urine dipstick test did not have microscopic haematuria at microscopy.4 Myoglobin and free haemoglobin in urine account for many of these false-positive results. Therefore, it is recommended by the American Urological Association that urological referral for asymptomatic patients should only be made if there are greater than three red blood cells per high power field in two of three urine analyses.5 It is also recommended that specialist referral should be initiated after a single positive urinalysis, if patients have symptomatic microscopic haematuria, gross haematuria or if they are considered to be at increased risk for urological malignancy; subgroups of patients which are at increased risk of urological malignancy include smokers, those with occupational exposure to benzene or aromatic amines, irritation on voiding, resistant urinary tract infections, pelvic irradiation and analgesic abuse.6 Patients with haematuria of suspected glomerular or tubular origin, suggested by the presence of proteinuria, red blood cell casts, raised creatinine or hypertension, should be evaluated by a nephrologist.7 The remaining patients, who are more likely to have an epithelial source of haematuria, should be reviewed by a urologist.7 A large number of lesions can give rise to haematuria; individual causes are discussed in greater detail in relevant chapters of this book. The most common non-glomerular causes of haematuria include stone disease, malignancy and infection. This chapter will assess aspects relevant to the radiological assessment of non-glomerular causes of haematuria (Table 34-1). TABLE 34-1 Non-glomerular Causes of Haematuria Divided on the Basis of Renal or Extrarenal Aetiology Uroradiological referral for patients with haematuria due to stone disease is common. Approximately 95% of patients with acute renal colic have haematuria.8 Pathological calcification can occur in the renal parenchyma or collecting system. Nephrocalcinosis refers to the presence of pathological calcification in the renal parenchyma; 95% of cases of nephrocalcinosis occur in the medulla and the remainder in the cortex. Medullary nephrocalcinosis is caused by hyperparathyroidism, medullary sponge kidney, renal tubular acidosis, hypervitaminosis D and primary hyperoxaluria.9 Cortical nephrocalcinosis is caused by acute cortical necrosis, chronic glomerulonephritis and primary hyperoxaluria. Nephrocalcinosis can be seen on conventional radiography and ultrasound. Stippled patterns of calcification are observed on conventional radiographs in the cortical and medullary regions of the kidneys due to cortical and medullary nephrocalcinosis, respectively. On ultrasound, increased echogenicity rather than discrete calcification may be perceived. Nephrolithiasis refers to stone disease in the renal collecting system. The incidence of nephrolithiasis in patients with microscopic and macroscopic haematuria is 7.8 and 8.8%, respectively.10 Calcium phosphate and calcium oxalate stones, which account for the majority of renal stones, are radio-opaque, as are struvite stones, which represent 15% of renal stones, and develop in the setting of infection and alkaline urine. Urate and xanthine stones represent 5% of renal stones and are radiolucent. Most renal stones are hyperattenuating on unenhanced computed tomography (CT) (150–1000 Hounsfield units (HU)). Crystalline stones are an exception; these are associated with use of protease inhibitors and are hypoattenuating. Calcification within tumours, haematoma, or fungal masses tends to have attenuation of less than 50 HU.11 Unenhanced CT or stone protocol CT is gradually becoming established as the one-stop imaging study of choice for renal and ureteric stone evaluation. Conventional radiography has a sensitivity of only 60% for renal stone detection, whereas ultrasound has a sensitivity and specificity for urolithiasis of 40 and 84%, respectively, compared with CT.12,13 Ultrasound tends to overestimate stone size and has reduced sensitivity and specificity for small stones and those located in the ureters.14 Meta-analysis of unenhanced CT compared with intravenous urography (IVU) also confirms superiority of CT for acute urolithiasis.15 CT radiation dose reduction techniques have dramatically reduced dose and strengthens the case in favour of CT as first-line imaging for urolithiasis. For example, adaptive statistical iterative reconstruction blended with filtered back projection can provide diagnostic imaging at approximately 20% of the normal radiation dose (Fig. 34-1).16 This is possible since most renal stones are conspicuous relative to surrounding tissues and therefore less radiation dose is required for diagnosis since a greater degree of image noise may be tolerated. This of course comes at the cost of potentially missing other lesions that have soft-tissue attenuation. CT is not only excellent for stone detection but also helps clinical decision-making. The presence and severity of ureteric or renal obstruction can be estimated and the likelihood of spontaneous stone passage may also be determined (Fig. 34-2). Spontaneous passage occurs in 76% of calculi that measure 2–4 mm, 60% for calculi that measure 5–7 mm, 48% for calculi that measure 7–9 mm and less than 25% for stones greater than 9 mm.17 CT is excellent for the detection and assessment of renal and urothelial tumours and is considered the imaging technique of choice for renal mass evaluation. CT imaging in this context is typically performed in the control, corticomedullary and nephrographic phases after intravenous contrast medium administration (Fig. 34-3). Unenhanced CT is useful for detection of calcification in renal tumours or fat in an angiomyolipoma (Fig. 34-4). Corticomedullary phase imaging is useful for cortical assessment, detection of angiogenesis in clear cell carcinoma, and recognition of pseudotumours, which may not be appreciated on later phase imaging. Most renal and urothelial tumours are maximally conspicuous during the nephrographic phase. A difference of greater than 20 HU between unenhanced and nephrographic phase units is strongly predictive of enhancement and therefore renal neoplasm, as is a Hounsfield unit difference of greater than 10 between the corticomedullary and nephrographic phases (Fig. 34-5).18 A difference of 10 HU between unenhanced and nephrographic phase CT is not indicative of enhancement, and a difference of 10–20 HU is equivocal. Increased attenuation can be observed in non-enhancing tissues after contrast administration due to a phenomenon known as pseudoenhancement, which is likely due to CT reconstruction algorithms. In addition, care must be taken when measuring enhancement, ensuring that the enhancing solid portion of a lesion is studied and not the cystic component (Fig. 34-6). The authors have a low threshold for biopsy in cases where imaging is equivocal and surgical management will be determined by whether a lesion is neoplastic. Unfortunately, oncocytomas cannot be accurately distinguished from renal cell carcinoma using CT, although it is suggested that magnetic resonance imaging (MRI) may be helpful for making this important distinction (Fig. 34-7). Contrast medium-enhanced MRI is also very useful for renal lesion characterisation, especially for detection of fat within an angiomyolipoma and diffusion- and perfusion-weighted imaging has also improved lesion detection and characterisation.19 CT assessment of haematuria can be performed using unenhanced CT and post-intravenous contrast medium-enhanced CT, including CT urography (CTU). CTU entails imaging the kidneys and collecting systems in the pyelographic phase. Normally, the collecting systems can also be examined in the control and nephrographic phases as part of a complete CTU protocol. The CTU technique is discussed in detail in Chapter 38. There is good evidence to indicate that traditional methods of haematuria assessment using IVU and ultrasound are improved upon by CTU. One compelling study compared retrograde ureteropyelography (UP), the imaging gold standard for assessing the ureters and renal pelvis, with CTU.20 A cohort of 106 patients who had initial assessment with IVU and cystoscopy underwent CTU and UP with a 3- to 5-year follow-up period and histological reference standard. The sensitivity, specificity, positive predictive value and negative predictive value of CTU for diagnosing transitional cell carcinoma of the upper urinary tract was 0.97, 0.93, 0.79 and 0.99, respectively. This was comparable to UP. Of note, the positive predictive value of CTU, 0.79, is not very strong. Patients should have histological confirmation prior to undergoing surgery for findings on CTU. One would prefer to use CTU for legitimate indications and minimise wasted resources and radiation exposure. A reasonable algorithm has been suggested to address this issue.21 Patients with haematuria could be divided into low- and high-risk groups following exclusion of urinary tract infection. Low-risk patients include patients with either microscopic or macroscopic haematuria aged less than 40 years and patients with microscopic haematuria aged greater than 40 years. High-risk patients include those with macroscopic haematuria aged greater than 40 years. Low-risk patients would be imaged with ultrasound if aged less than 40 years for the assessment of medical renal disease and non-contrast CT would be used for patients aged less than 40 years with macroscopic haematuria or patients aged greater than 40 years with microscopic haematuria, since renal stone disease is the most common cause of haematuria in these patients. Patients in the high-risk group would be imaged with CTU. It has also been suggested that a risk stratification score could be incorporated into this algorithm to ensure optimal patient selection for CTU. Similarly, CTU can be used as a triage tool for intravesical transitional cell carcinoma assessment in the setting of haematuria. Based on a large study of 778 patients with visible haematuria aged >40 years, it was suggested that, rather than investigating all patients initially with flexible cystoscopy, patients should first undergo CTU. If a bladder lesion is detected by CTU, patients should undergo rigid cystoscopy, whereas a normal CTU should be followed with flexible cystoscopy. The authors suggested that following this strategy would reduce the number of flexible cystoscopies by 17% without reducing detection rates.22 Urine cytology has had a poor sensitivity and some suggest that it no longer has a role at a rapid diagnosis clinic where CTU and cystoscopy are available. Although CT has superseded IVU and ultrasound for detection of urothelial cancers, many of the imaging signs sought on CT imaging have been previously described using IVU.23 Early urothelial neoplasms appear as subtle filling defects or focal mural thickening on IVU and CT (Fig. 34-8). Transitional cell cancers (TCC) can appear as fixed smooth or irregular, single or multiple filling defects within the renal collecting systems. Renal TCC can obstruct an infundibulum, creating a phantom calyx that may or may not fill with contrast (Fig. 34-9). Pelviureteric junction obstruction can produce a delayed nephrogram or ureteric involvement can cause acute hydronephrosis. Signs of ureteric TCC include absent renal function, eccentric or circumferential fixed wall thickening, filling defects, hydronephrosis with or without hydroureter and irregular ureteric narrowing with proximal shouldering termed the goblet sign (Fig. 34-10). Filling detects may also be produced by metastases, calculi, blood clots, infection, a vascular impression or congenital abnormality such as pyelourethritis cystica. The ultrasonic characteristics of urothelial tumours are variable. Transitional cell cancer is often slightly hyperechoic, but may be difficult to differentiate from renal sinus fat, and further imaging is always needed for confirmation. There are many other causes of haematuria. Infection will be discussed later in this chapter. Many causes are diagnosed early in life using ultrasound, based on family history, or clinical findings, such as von Hippel–Lindau syndrome, tuberous sclerosis, polycystic kidney disease and medullary sponge kidney. Repeated imaging plays an essential role in screening for renal tumour development in many of these cases. It is preferable in many cases that this be done using MRI if possible since contrast resolution will be superior to unenhanced CT in patients with renal insufficiency, and, even in the absence of renal insufficiency, the use of MRI will limit the number of CT studies performed over a patient’s lifetime and lifetime cumulative radiation exposure (Fig. 34-11). Haematuria as a result of trauma requires imaging to determine whether there is renal parenchymal injury, involvement of perirenal or periureteric tissues, bladder rupture and/or presence of pseudoaneurysm. Unenhanced imaging as part of CT evaluation in the setting of suspected trauma is essential. When performing angiography in the setting of iatrogenic haematuria following percutaneous nephrostomy drain insertion, an angiographic run may need to be performed while the nephrostomy drain is removed over a guidewire, to detect active bleeding or pseudoaneurysm formation, when not seen on initial angiographic sequences (Fig. 34-12). The loin or flank is an anatomical area between the ribs and pelvis lateral to the spine. The kidneys and ureters receive visceral nerve supply from T10–L1 and T11–L1, respectively. Renal or ureteric pathology causes referred painful stimuli transmitted by the somatic fibres to be perceived over the dermatomes at the loin. Acute flank pain is a common clinical entity which may be secondary to urinary or extraurinary causes.24 Urinary obstruction secondary to urinary tract calculi is the most common cause. Urinary tract calculi are very common, affecting 3–5% of the population,25 men more than women, and the incidence increases with age up to 60 years.23 Caucasian patients are more likely to develop urinary tract calculi than patients of African origin and calculi are much less common in children than adults.23 Calculi typically become lodged in specific anatomic areas along the course of the ureter: the ureteropelvic junction, where the ureter crosses the iliac vessels, and at the vesico-ureteric junction.26 It is important to remember that the probability of spontaneous passage of small calculi (less than or equal to 5 mm) is very high but that larger stones (over 10 mm) are unlikely to pass spontaneously.25 Therefore, determination of the size of a ureteric calculus is fundamental to deciding the appropriate management. An ideal imaging investigation for patients with acute flank pain should be a single imaging test that provides accurate information not only about the present or absence of urinary tract stones but also about the size and position of the calculus and the presence of related complications, such as obstruction. One of the major clinical challenges managing patients with urinary tract calculi is that symptoms are frequently non-specific and also that urinary tract calculi tend to be recurrent. In addition, there are many other potential causes of acute loin pain. Other urological causes of loin pain include blood clots or tumour in the collecting systems, obstruction of the pelviureteric junction and infection such as acute pyelonephritis or pyonephrosis. There are several non-urological causes of acute loin pain including gallbladder problems such as biliary colic or acute cholecystitis, abdominal aortic aneurysm leakage, pneumonia, myocardial infarction, ovarian or testicular torsion, ectopic pregnancy, appendicitis, omental infarction, epiploic appendigitis, inflammatory bowel disease, acute diverticulitis or even intervertebral disc prolapse. Chronic loin pain can be caused by urological neoplasms including renal cell carcinoma, transitional cell carcinoma, renal and ureteric stones, tuberculosis, pelvic ureteric junction obstruction, testicular cancer, gastrointestinal neoplasms and spinal disease. Therefore, evaluation with imaging is recommended at initial presentation. The evaluation of acute flank pain commonly includes urinalysis, plain radiography of the kidneys, ureter and bladder (KUB), intravenous urography, ultrasound, or unenhanced CT (known as stone protocol CT or CT KUB) used alone or in combination.25 The role of MR urography in this setting is still being studied, but limited sensitivity for calcification remains a major limitation. Plain radiography is still often the first imaging study performed for a patient with loin pain. The sensitivity of plain radiography for detection of urinary tract calculi has been reported in the range 58–62%. Using CT as a reference standard, Levine et al. reported sensitivities of only 59% for detecting urinary tract calculi on KUB.27 Currently the role of KUB is limited when performed alone, but may be very useful when used in combination with ultrasound for detection of urinary tract calculi. However, even when KUB is used in combination with ultrasound, their combined sensitivities are well below unenhanced CT.25 Ultrasound in the setting of acute flank pain may be very useful. First, exposure to ionising radiation is avoided, which is especially important in children and pregnant patients. Ultrasound is capable of determining whether there is obstruction of urinary tract by assessing for direct signs of obstruction such as hydronephrosis and hydroureter. Early ultrasound in this setting, however, can miss acute obstruction in up to 30% of patients since it can take several hours for hydronephrosis and hydroureter to develop following stone impaction.25 Ultrasound suffers further disadvantages in pregnant patients due to the phenomenon of physiological hydronephrosis, which is observed in up to 80% of patients. This occurs in the absence of urinary stones and affects the right side more often than the left. Neither ultrasound, IVU or MR urography is as sensitive or specific as CT for determining the cause of obstruction. Combined imaging using ultrasound to determine the presence or absence of hydronephrosis and hydroureter in conjunction with KUB to examine for urinary tract calculi is a common imaging strategy in the setting of acute loin pain. This remains less sensitive for urinary tract calculus detection compared with IVU and is much less sensitive than unenhanced CT (stone protocol). MR urography in the evaluation of patients with acute flank pain is limited by poor access in the acute setting at many centres, increased study duration compared with unenhanced CT, and most importantly, by substantially reduced sensitivity for urinary tract stones and, therefore, for definitive direct stone identification.28 Differentiation of a urinary stone from other intraluminal filling defects such as blood clot or tumour is difficult on MR urography. MR urography is frequently used in pregnant patients with loin pain and suspected obstructing urinary tract calculi. This is often performed following ultrasound of the kidneys. MR urography in this setting is useful for confirming the presence of true hydronephrosis and determining the level of apparent obstruction. As with MR urography in the non-pregnant patient, definitive detection of renal and ureteric calculi is difficult because of the limited sensitivity for calcification. Recent advances in low-dose CT have shown good sensitivity and specificity for stone diagnosis in pregnant patients, but the use of low-dose CT in this setting remains unproven and still very controversial. Since Smith et al. first described the use of unenhanced CT or stone protocol CT in evaluation of the patients with loin pain, multiple studies have confirmed impressive sensitivities of 95–96% and specificities of 98% for detection of urinary tract calculi.29 Not only is unenhanced CT good for determining the presence of calculi but also it can detect associated obstruction and also immediately determines the level of obstruction and the size of the obstructing stone. Obstruction is suggested by identifying hydronephrosis and hydroureter above the level of the obstructing calculus and also by the presence of secondary signs of obstruction such as perirenal and periureteric fat stranding. Indeed, the degree of perinephric fat stranding in the obstructed kidney has been reported in some studies to correlate inversely with the likelihood of stone passage. Unenhanced CT also enjoys the advantage of contrast medium avoidance over IVU. CT is rapid and does not require repeated imaging (compared with IVU) to determine the site of obstruction. The major concern associated with the use of unenhanced CT, however, is associated exposure to ionising radiation. In recent years, unenhanced CT protocols which can provide comprehensive evaluation of the urinary tract for the presence of calculi with effective doses reported in a range comparable to IVU or even lower have been described. A meta-analysis of studies assessing diagnostic accuracy of low-dose unenhanced CT for evaluation of patients with suspected urinary tract calculi in the sub 3-mSv range found sensitivities of 97% and specificity of 95% for detection of urinary tract calculi.25,30 Recently, there has been increasing interest in the use of dual-energy CT as a means of characterising urinary tract calculi composition. Some studies have suggested that dual-energy CT imaging can be used to differentiate various subtypes of urinary tract calculi such as uric acid stones, cysteine stones and struvite stones.31 Before the development of unenhanced CT protocols for suspected urinary stones, IVU was the investigation of choice in this setting.25 There are several reasons why IVU has been replaced by CT: avoidance of the requirement for intravenous contrast medium administration with CT, avoidance of repeated studies up to 24 hours after commencing IVU, and better determination of the site of ureteric obstruction using CT. Another major advantage of CT is the ability to detect alternative causes of flank pain such as acute appendicitis, acute diverticulitis, epiploic appendagitis and others which can be difficult to diagnose with ultrasound and are almost never identified de novo on IVU and plain radiography. The most important imaging finding on CT in acute ureteral obstruction secondary to urinary stone disease is direct visualisation of a calculus in the ureteric lumen. The certainty of diagnosis is strengthened by finding a dilated ureter above the stone. Definitive characterisation of a calcification as a calculus is supported by the identification of a soft-tissue rim sign.23 This is a soft-tissue attenuation surrounding the calcification and indicates that the calcification is in the ureter. Pelvic phleboliths do not have surrounding soft tissue and therefore this sign is crucial for distinction. The presence of increased perinephric or pereuretericfat stranding also represents a secondary sign suggestive of downstream ureteric obstruction. There are also a number of potential pitfalls on unenhanced CT in the detection of urinary tract calculi and in the characterisation of an abdominal or pelvic calcification as a urinary tract calculus. Calcifications of iliac vessels and their branches can be difficult to differentiate from an adjacent ureteric calculus.23 When it is difficult to definitively conclude that an abdominal or pelvic calcification lies in the lumen of the left ureter, intravenous contrast medium administration and pyelographic phase CT imaging can help make the distinction between ureteric calculi and extraluminal calcifications.23 Currently, this strategy carries a disadvantage of increased exposure to ionising radiation and potential nephrotoxicity from iodinated contrast media. Other problems for radiologists on unenhanced CT include parapelvic cysts or an extrarenal pelvis which can mimic hydronephrosis;23 the distinction of these entities can be made by ultrasound or on pyelographic phase enhanced CT. A large adnexal cyst can simulate a distended bladder. Following the entire ureteric course on unenhanced CT in a thin patient with paucity of peritoneal and retroperitoneal fat can be very difficult and this situation can be exacerbated by the present of multiple phleboliths in the pelvis. Imaging of the kidneys in the setting of renal failure has two principal aims: to demonstrate or exclude obstruction and to show renal size, since small kidneys indicate irreversible chronic renal failure. Ultrasound is generally the first imaging investigation performed in this clinical setting, since it assesses renal size and collecting system appearances. Underlying causes of renal impairment such as autosomal dominant polycystic kidney disease or collecting system stones can also be identified during the course of this examination. Renal obstruction is demonstrated on ultrasound in an indirect manner by showing pelvicalyceal and/or ureteric dilatation proximal to the level of obstruction, with a change in calibre of the collecting system and/or ureter at the level of obstruction which is often not well seen using ultrasound alone. Obstructive appearances can be difficult to distinguish from non-obstructive lesions such as an extrarenal pelvis or parapelvic cyst. The Doppler resistive index ((peak systolic − end diastolic velocity)/peak systolic velocity) measure is a useful means of differentiating acute renal collecting system obstruction from non-obstructive causes of collecting system dilatation. The resistive index is greater than normal (0.7) in the setting of acute obstruction. Ultrasound can help differentiate between the two most common causes of acute renal failure. An increased resistive index is observed in non-obstructive renal failure due to acute tubular necrosis but not in the setting of prerenal azotaemia.32 Unenhanced CT is indicated to assess the pelvicalyceal system if ultrasound is inconclusive, to detect stones or as part of a multiphase study. Unenhanced MRI may be used as an alternative to CT and provides similar anatomical information, with coronal T2-weighted sequences providing detailed imaging of the collecting system. Dynamic radionuclide renography is the simplest and safest way to assess renal perfusion in patients with renal failure, helping to differentiate reversible causes such as acute tubular necrosis from other conditions with a poorer prognosis such as renal cortical necrosis. Ultrasound is also a practical means of image guidance for non-focal biopsy in the setting of renal dysfunction. Imaging-guided renal biopsy is indicated for patients with unexplained acute renal failure. Haemorrhage is one of the potentially serious complications of renal biopsy. Renal biopsy is considered a high-risk procedure in terms of bleeding risk since bleeding can be hard to detect and control.33 Asymptomatic perinephric haematoma is demonstrated in up to 90% of patients on CT and 44% on ultrasound following renal biospy. Asymptomatic arteriovenous fistulae also occur but these often spontaneously close.34 Symptomatic bleeding, macroscopic haematuria or perinephric haematoma occur in less than 10% of patients after biopsy and less than 5% of these patients require transfusion or surgery. Mortality rate of renal biopsy is approximately 1 per 1000. Small kidneys with a parenchymal thickness of 1 cm or less are not usually biopsied because histological samples have a greater chance of being non-diagnostic and biopsy has an increased associated complication rate in these cases. Renal size and parenchymal thickness are used to categorize non-obstructive renal failure as acute or chronic; chronic renal failure is characterised by small kidneys with thin parenchyma. Ultrasound provides accurate measurements of renal length, although this is normally less than the corresponding urographic measurement, with 9 cm representing the lower limit of normal adult kidney length, equivalent to a urographic measurement of 11.5 cm. Renal parenchymal thinning occurs before renal length decreases. Chronic kidney disease is divided into five stages: Stage 1: normal (glomerular filtration rate > 90 mL/min/1.73 m2). Stage 2: mild impairment (glomerular filtration rate 60–89 mL/min/1.73 m2). Stage 3: moderate (glomerular filtration rate 31–59 mL/min/1.73 m2). Stages 4 and 5: severe (glomerular filtration rate 15–29 and < 15 mL/min/1.73 m2, respectively).35 In non-obstructive chronic renal failure, ultrasound demonstrates small kidneys with diffuse or focally reduced parenchymal thickness. As in acute renal failure, parenchymal reflectivity may be normal or increased, and increased reflectivity does not point towards a specific diagnosis. Although an increased resistive index (RI) may be detected in the intrarenal arteries on Doppler interrogation, this is often insufficient alone to identify the cause of renal failure. Ultrasound elastography for renal assessment is currently being investigated as a means of quantifying renal fibrosis. The most common cause of chronic kidney disease is diabetes. Non-diabetic causes are divided into glomerular, vascular, tubulointerstitial and cystic aetiologies. Notable causes of renal failure relevant to imaging include the following conditions. Autosomal dominant polycystic kidney disease is responsible for renal failure in 10–15% of patients who receive dialysis. Between 5 and 15% of patients with tuberous sclerosis develop renal failure and have multiple cysts with an appearance indistinguishable from autosomal dominant polycystic kidney disease. Differentiation depends on detecting the other typical clinical features of tuberous sclerosis (e.g. central nervous system features). Acquired cystic kidney disease is observed in up to 13% of patients with chronic renal disease before commencing dialysis.36 It also occurs in patients on long-term haemo- or peritoneal dialysis and depends on the duration of dialysis, occurring in over 90% of patients after 5 years of dialysis. The majority of patients are asymptomatic but in some patients cysts bleed and cause pain or haematuria. ACKD can usually be distinguished from autosomal dominant polycystic kidney disease because the kidneys are small or normal in size and cysts are less numerous. Patients with ACKD have an increased incidence of renal cell carcinoma, including low-grade papillary tumours. Following renal transplantation, ACKD persists and the risk of haemorrhage and development of renal cell cancer also continues. Renovascular disease is an important cause of chronic renal failure, responsible for renal failure in 14% of patients over the age of 50 years old accepted for renal replacement therapy and, more importantly, it represents one of the few treatable causes. Doppler resistive index is increased in patients with non-obstructive causes of renal failure. A decreased resistive index can be observed in patients with flow-limiting renal artery stenosis and can cause a parvus tardus wave pattern due to reduced arterial flow, and a resistive index difference of more than 0.05 between kidneys helps in the identification of renal artery stenosis. Overall, resistive index measurements are not specific for renal artery stenosis detection, since the index can also be increased by renal vein thrombosis and renal obstruction. Cross-sectional imaging by CT or MR angiography should be considered for confirmation in these cases. Contrast medium nephrotoxicity is defined as a serum creatinine increase of more than 25% or 44 µmol/L within 3 days of intravascular contrast medium administration, for which there is no other explanation.37,38 The causality link between intravenous contrast medium administration and nephropathy has received renewed interest recently. This is based on the observation that many assumptions have been made when extrapolating results from invasive angiographic studies which lacked control arms and that 13 control studies showed that patients who received contrast medium were less likely to suffer acute kidney injury.39
Common Uroradiological Referrals
Haematuria, Loin Pain, Renal Failure and Infection
Haematuria
Renal
Extrarenal
Tumour (renal cell cancer, transitional cell cancer angiomyolipoma, oncocytoma)
Tumour (transitional cell and prostate cancer, benign prostatic hyperplasia)
Vascular (arteriovenous malformation, renal vein thrombosis, infarct, transplant rejection, malignant hypertension, sickle cell disease)
Renal or ureteric stones
Metabolic (hypercalciuria, hyperuricuria)
Infection (cystitis, prostatitis, urinary schistosomiasis, tuberculosis, condyloma acuminatum)
Familial (polycystic kidney disease, medullary sponge kidney)
Coagulopathy or bleeding diathesis
Infection (pyelonephritis, tuberculosis, cytomegalovirus, infectious mononucleosis)
Trauma (including catheterisation and radiation)
Papillary necrosis
Medications (heparin, warfarin, cyclophosphamide)
Renal Tract Calcifications
Imaging of Renal and Ureteric Stones
Imaging of Renal and Ureteric Tumours
Risk Stratification of Patients with Haematuria for Triage to Computed Tomography
Imaging Features of Urothelial Tumours on Computed Tomography
Loin Pain
Imaging of Patients with Loin Pain
Radiography
Ultrasound
MR Urography
Computed Tomography
Intravenous Urography
Useful Signs on Unenhanced CT and Potential Pitfalls
Renal Failure
Chronic Renal Failure
Autosomal Dominant Polycystic Kidney Disease (Adult Polycystic Kidney Disease)
Tuberous Sclerosis
Acquired Cystic Kidney Disease (ACKD)
Renovascular Disease
Contrast Medium-Induced Nephrotoxicity
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Common Uroradiological Referrals
Chapter 34