Muscular dystrophy is a group of genetically inherited diseases with irreversible and progressive muscle loss and is associated with cardiac involvement. Particularly in Duchenne and Becker dystrophies, cardiac disorders are the leading causes of mortality. Cardiovascular magnetic resonance imaging (CMR) can detect even incipient myocardial fibrosis (late gadolinium enhancement), which has prognostic significance in patients with preserved left ventricular function by echocardiogram and before the onset of symptoms. Early detection of cardiac abnormalities by CMR enables early cardioprotective treatment, leading to a better prognosis.
Key points
- •
Muscular dystrophies are myogenic muscle disorders characterized by degradation and loss of function, with variable distribution and severity.
- •
Cardiac involvement is manifested predominantly as hypertrophic, dilated, and arrhythmic cardiomyopathy, with high morbidity and mortality.
- •
Cardiovascular MR imaging may be of considerable value for early detection of cardiac disease before the onset of cardiac symptoms and electrocardiography and echocardiogram abnormalities.
Introduction
Muscular dystrophies are myogenic muscle disorders characterized by degradation and loss of function, with variable distribution and severity. They are caused by mutations in genes encoding various proteins responsible for muscle contraction and consequent muscle weakness and progressive loss of function. According to the pattern of inheritance and localization of muscle weakness, they can be classified as specific muscular dystrophies. The most prevalent dystrophies commonly associated with cardiac complications include Duchenne, Becker, and female patients carrying mutations in the dystrophin gene; and limb-girdle, Emery-Dreifuss, and myotonic muscular dystrophies. These specific muscular dystrophies and their cardiac involvement phenotypes assessed by cardiovascular MR (CMR) imaging are described in Table 1 .
| Dystrophy | Clinical Features | CMR Imaging Features |
|---|---|---|
| DMD | Age of onset: 3–7 y | Subepicardial fibrosis of the inferolateral wall or transmural LGE |
| BMD | Age of onset: teenage years | |
| Female DMD or BMD mutation carriers | Cardiac screening has been recommended after the teenage years | Myocardial fibrosis similar to DMD patients, including transmural LGE |
| EDMD | Bimodal distribution: first or second decade, sometimes adult onset | CMR imaging data are limited Normal myocardium is replaced by fibrous and adipose tissue, usually starting in the atria Myocardial fibrosis does not necessarily precede systolic dysfunction Midwall LGE may occur in the basal or mid ventricular septum segments |
| LGMD | Variable onset (early childhood to adulthood) | AD subtype: midwall LGE of the basal interventricular septum well before the onset of LV dilatation and systolic dysfunction LGMD2I: midwall or subepicardial fibrosis of the septum and inferior wall (extensive lateral wall LGE has also been described) |
| MD | MD1
Early childhood to adulthood onset,
rarely during infancy (congenital form) MD2 adult onset, usually fourth decade | MD patients may present cardiomyopathy (usually more benign in MD2 than in MD1), dilatation, systolic dysfunction, hypertrophy, and (occasionally) noncompaction LGE data are limited, although septum and basal inferolateral midwall fibrosis has been reported |
Cardiac involvement is manifested predominantly as hypertrophic, dilated, and arrhythmic cardiomyopathy, with high morbidity and mortality. Arrhythmias are very frequent and may manifest with the event of the sudden cardiac death. Patients with a molecularly confirmed diagnosis of Duchenne muscular dystrophy have a median survival of 24 years. The cardiac involvement detected by diagnostic imaging usually precedes the clinical symptoms, considering the limited mobility of these patients. With the respiratory care improvement, cardiac disorders have become the leading cause of mortality in many of these patients. The early detection of cardiac involvement also enables early cardioprotective treatment with consequent reduction of the cardiac remodeling and attenuation of heart failure symptoms, which is presented in several recently published studies, mainly in dystrophinopathies.
Electrocardiogram (ECG) and echocardiogram are routinely used in the evaluation of these patients; however, in many of these diseases, their ability to detect early and subclinical involvement of the heart muscle is limited. CMR imaging has contributed to the early diagnosis of cardiac involvement in various muscular dystrophies, allowing early treatment and possibly reduction of mortality.
Muscular dystrophies related to dystrophinopathies
Duchenne and Becker muscular dystrophies are genetically determined diseases caused by a mutation in the dystrophin gene located on the X chromosome Xp21 locus. Dystrophin connects the muscle fiber cytoskeleton with the extracellular matrix and is responsible for the stability of muscle fibers during the muscular contraction. With reduced amount (Becker) or absence (Duchenne) of dystrophin occur successive sarcolemma microruptures during muscle contraction, with the consequent necrosis and replacement of the fibers by adipose and fibrous tissue. In DMD, after a short motor development period that is apparently normal, there is progressive and irreversible muscle degeneration with consequent muscle weakness.
Duchenne muscular dystrophy (DMD) is the most common of all muscular dystrophies (1 out of 3000–5000) and BMD is 10 times less frequent.
In the natural history of these dystrophies, there is involvement of skeletal muscle groups, such as the lower members and respiratory muscles, and also the myocardium. Symptoms of heart failure may be nonspecific, such as fatigue, weight loss, vomiting, and sleep disorders. These patients often develop arrhythmias and may have complete atrioventricular (AV) block associated with severe systolic dysfunction in the final stage of the disease. The main cause of death is respiratory and/or heart failure. However, with the optimization of respiratory care, the main cause of death has been reported to be of cardiac origin.
An approach for early diagnosis of cardiac involvement of these dystrophies is essential to maximize the length and quality of life. Even clinical data such as early diagnosis of blood pressure changes can trigger useful appropriate therapy potentially leading to increased life expectancy. More specifically, echocardiography is routinely used in the diagnosis of cardiomyopathy. Approximately 28% of patients with DMD exhibit cardiac abnormalities detectable by echocardiogram at the age of 14 years and 57% by the age of 18 years. In DMD patients, an echocardiogram may be limited by a poor acoustic window due to kyphoscoliosis associated with disease progression and the chest adiposity in most patients from the chronic use of corticosteroids. In contrast, CMR imaging is reproducible and allows full access to the evaluation of global and regional ventricular function (a gold standard method) and myocardial tissue characterization.
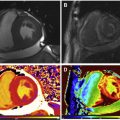
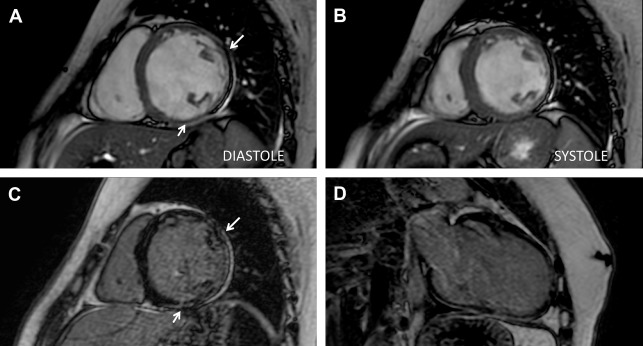
Previous studies showed that cardiac involvement is insidious and precedes the onset of symptoms of heart failure, occurring usually after adolescence. In 2007, Silva and colleagues reported, for the first time, the presence of myocardial fibrosis (MF) detected by CMR imaging in subjects with DMD and BMD who had no clinical signs of cardiac involvement or cardiac alterations detectable by conventional echocardiogram, chest radiograph, and ECG. Seven of 10 subjects (70%) had MF. Nonischemic late gadolinium enhancement (LGE) with midwall and subepicardial involvement of the left ventricular (LV) lateral segments was the most frequent pattern of fibrosis (89%): midwall in 57.8% and subepicardial in 31.1% ( Fig. 1 ).
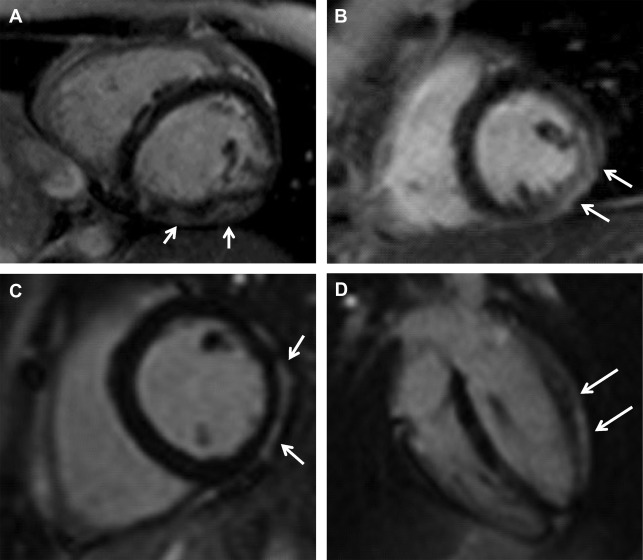
In subjects with systolic dysfunction, the agreement between the segmental MF extent and the degree of segmental dysfunction was fair (kappa 0.31, P <.001), emphasizing the importance of fibrosis in the development of cardiomyopathy. Interestingly, MF was detected in very young subjects: in 2 of 4 subjects younger than 10 years old and with a normal echocardiogram. Moreover, of 7 subjects with MF, only 2 had abnormal echocardiography.
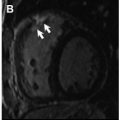
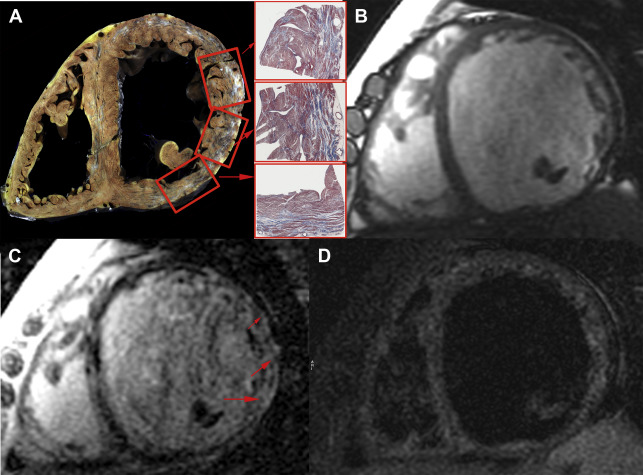
LGE is considered the gold standard noninvasive method to detect MF, reaffirming the importance of CMR imaging to detect cardiac involvement and guide early treatment of a variety of cardiomyopathies, and has also been advocated as such in muscular dystrophies. In the specific case of BMD, Fig. 2 presents an autopsy case with macroscopic and histopathological validation of LGE by CMR imaging accurately indicating the areas with MF by Masson trichrome stain. To the authors’ knowledge, this is the first figure demonstration of such a cross-correlation between LGE by CMR imaging and MF by pathologic assessment in BMD (see Fig. 2 ).
A randomized clinical trial was conducted by Silva and colleagues in 2 centers, and included 76 male subjects with DMD or BMD (mean age at baseline ∼13 years) undergoing 2 CMR imaging studies with a 2-year interval for ventricular function and MF assessment. In a non–intent-to-treat trial, 42 subjects with MF and normal LV ejection fraction (LVEF) were randomized (1:1) to receive or not receive angiotensin converting enzyme (ACE) inhibitor therapy. Chest radiography, ECG, and echocardiogram were also carried out in this period.
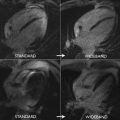
MF was present in 72% of the subjects and LV systolic dysfunction in only 24% of them. The pattern of MF was mostly midwall and subepicardial, predominantly affecting lateral and inferior LV segments ( Fig. 3 ).
MF at baseline was an independent predictor of lower LVEF at follow-up ( P = .03). Among subjects with MF and preserved ejection fraction, those randomized to receive ACE inhibitors demonstrated slower MF progression compared with the untreated group ( P = .001), even though all groups have had MF progression.
There was a significant positive correlation between age and the amount of MF identified by CMR imaging, both at baseline and at follow-up (correlation coefficients, r = 0.52 at baseline and r = 0.50 at follow-up; P <.001 for both). Moreover, all the studied age groups presented MF and progression up to the age of 15 to 16 years, and slower progression after that.
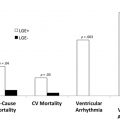
Subjects with MF noted on CMR imaging had a higher probability of cardiovascular events ( P = .04).
In multivariate analysis, ACE inhibitor therapy was an independent indicator of decreased MF progression ( P = .04). This study demonstrated that the use of ACE inhibitors in subjects with DMD and BMD diagnosed with MF by CMR imaging caused a reduction of the progression of MF in a period of 2 years. These findings reinforce the need for early diagnosis and treatment of cardiac involvement in subjects with DMD and BMD before the onset of symptoms. ECG, echocardiogram, and chest radiograph showed low sensitivity and negative predictive value for early detection of cardiac involvement in DMD and BMD, reinforcing the usefulness of CMR imaging in this clinical setting.
A key aspect in cardiac involvement in BMD or DMD patients is the genetic profile. Jefferies and colleagues showed a strong association between mutations in exons 12 and 14 to 17 with LV dysfunction. Additionally, the investigators showed that mutation in exons 51 to 54 and 68 to 71 had a possible cardioprotective effect. Other studies pointed out that some dystrophin isoforms are absent in the myocardium and are transcripted in exons greater than 45 and 56. A randomized trial by Silva and colleagues was the first to demonstrate that subjects with mutations in exons less than 45 had greater amounts of MF and lower LVEF in both basal and follow-up CMR imaging studies. There was a significant correlation between the site of mutation in the dystrophin gene and MF. Mutations in exons greater than or equal to 45 appear to protect against cardiac involvement. However, the impact of these findings on clinical management remains to be determined.
Ashford and colleagues studied subjects with DMD and demonstrated subclinical cardiac dysfunction using the myocardial tagging technique of CMR imaging with alteration of segmental contractility in the lateral and inferior walls.
A clinical trial was conducted by Raman and colleagues that enrolled boys aged 7 years or older with DMD. It was demonstrated that, at an early stage of myocardial disease (myocardial damage by LGE CMR imaging and preserved ejection fraction), the addition of eplerenone to background ACE inhibitors or angiotensin receptor blocker (ARB) therapy attenuates the progressive decline in LV systolic function (LV circumferential strain; P = .020).
A working group of the National Heart, Lung, and Blood Institute (NHLBI) published an update (NHLBI 2015) on the cardiac involvement in DMD. CMR imaging was considered as a noninvasive modality of choice except in young patients who do not cooperate with the maneuvers necessary to accomplish the examination. Echocardiogram should be performed until the age of 6 to 7 years. After this, at least 1 CMR imaging should be performed every 2 years, and annually after 10 years when the risk increases of cardiac involvement (MF, dilated left ventricle and ventricular dysfunction). Traditionally, ACE inhibitors and ARBs have been used as first-line treatment in cardiac patients with DMD or Becker muscular dystrophy. The NHLBI 2014 recommends the use of ACE inhibitors or ARBs in patients 10 years or older with DMD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree