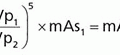Computed Tomography
10.0 INTRODUCTION
Computed tomography (CT) has experienced enormous growth in clinical use over the past three decades primarily due to significant advances in image quality and a dramatic reduction in acquisition time as the technology has advanced. Image quality has increased because of better detector sampling along the long axis (z-dimension) of the patient with multiple detector array systems of 40 to 160 mm beam coverage in one rotation. CT reconstruction has advanced from filtered backprojection (FBP) to generations of iterative reconstruction and deep learning (DL)-based adjuncts, providing lower noise images at lower radiation dose levels for improved spatial resolution and contrast resolution. With half-second gantry rotation (or shorter), 80 mm of patient length can be acquired in 1 second (s), and hence 400 mm of patient length can be scanned in 5 s.
CT imaging has gained widespread use across many clinical applications for abdominal, thoracic, head, musculoskeletal, CT angiography (CTA), organ-specific CT perfusion, and dual-energy CT. Thin-slice axial images, multiplanar reconstruction, and 3D volume rendering reconstruction allows segmentation of bone structures from soft tissue, accurate assessment of iodinated contrast in CT angiograms, and the ability to use volumetric data for 3D printing. Coronal and sagittal CT visualization provides important additional information to the interpreting physician, for spinal alignment, orientation of pathology, and normal anatomy including identifying feeding vessels, gastrointestinal tract topography, abdominal organ placement and orientation, trauma, and other factors.
1. Acquisition geometry—x-ray tube rotates in a gantry around the long axis of the body
a. For one rotation, multiple projections are acquired and reconstructed into axial images.
b. Two-dimensional images are comprised of pixels with equal dimensions in horizontal (x) and vertical (y) axes.
d. Dimensions of each voxel are on the order of 0.6 mm (Δx and Δy) by 0.5 mm (Δz).
e. A 75-kg person can be imaged with over 400 million voxels using CT.
2. Volume data set and multiplanar reconstruction from thin-slice axial images (Fig. 10-2)
a. Routine reformatting into the coronal and sagittal planes provide intuitive anatomical display.
10.1 BASIC CONCEPTS
1. X-ray tube potential for routine scanning—120 kV; other tube voltages also used
a. Tube voltage is varied to optimize image quality versus radiation dose for clinical applications.
b. Protocols and selection of voltage are based on patient size and clinical need or diagnosis.
c. Tube voltage options (typical): 80, 100, 120, 140 kV (Fig. 10-3).
(i) Added filtration in beam creates a “hard” x-ray spectrum.
(ii) Physical properties (attenuation) of tissues are based on these spectra.
d. Effective energy for kV spectra range from approximately 43 to approximately 70 keV (Fig. 10-4).
e. Compton scatter is 10-fold more likely than photoelectric or Rayleigh scatter interactions for soft tissue.
f. For bone and iodinated contrast agents, the photoelectric interaction does play an important role.
2. Attenuation coefficient for Compton interactions, Equation 10-1:

where N is Avogadro’s number (6.023 × 1023), Z is the atomic number, and A is the atomic mass
a. For most elements (except H), Z/A ratio is ½; density tends to dominate when comparing adipose-rich tissues (lower density) to soft tissues in terms of attenuation at these energies.
3. The Hounsfield unit (so named after a principal developer of CT, Sir Godfrey Hounsfield)
a. Preprocessing of CT acquired data applies a logarithmic function to the ratio of incident to transmitted x-ray intensity: ln ; this effectively linearizes the effective attenuation coefficient.
; this effectively linearizes the effective attenuation coefficient.
b. Subsequent reconstruction provides grayscale encoding (Eq. 10-2), a function of the linear attenuation coefficient, called the Hounsfield unit (HU).

For a given voxel in the image, K, which contains average µK, the HU is scaled to µw, the linear attenuation coefficient of water.
c. The HU is defined at water (µK = µw); HUwater = 0, and air (µK = µair); HUair = -1,000.
d. µ has relatively strong dependencies on x-ray beam energy as µ(E).
(i) The notation for µ in Equation 10-2 represents the effective linear attenuation coefficient, µeff.
(ii) Calibration of HU is slightly different at each tube voltage, but for water HU = 0 at all tube voltages; other tissues such as liver and bone will have slightly different HU values at different tube potentials.
e. Imprecision in HU values results from calibration, scattered radiation, quantum noise, and beam hardening.
f. Typical HU for water is usually within ±5 HU with often larger deviations for other tissues and air.
10.2 CT SYSTEM DESIGNS
1. The gantry—geometry and detector configuration
a. Clinical CT scanners have the x-ray source and detector arrays arranged in an arc (Fig. 10-5).
b. Detector arrays aligned along a radius of curvature provide perpendicular incidence of the beam.
c. A fan-beam projection incident on individual detectors.
(i) For a given view (tube position and view angle), the individual x-rays (rays) define a line integral extending from the source to each individual detector along the detector array.
(ii) The collection of all rays across the detector elements constitutes a view or projection.
(iii) Raw data collected during a scan are comprised of rays and views.
d. Basic geometry of a CT scanner and dimensions (Fig. 10-6).
(i) Isocenter of rotation is almost always the center of the reconstructed image.
(ii) For source to isocenter distance A, and source to detector distance B, magnification M = B/A.
(iii) Detector width and length specified at isocenter: if B = 95 cm and A = 50 cm, M = 1.9 and detector width specified as 0.5 mm has an actual physical width of 1.9 × 0.5 = 0.95 mm.
(iv) Fan angle of the x-ray beam is approximately 50° to 60° and defines maximum scan FOV.
e. Multidetector array CT scanner (MDCT) geometry (Fig. 10-7).
(i) 16, 64, 256, or more detector arrays are abutted to create a larger sampling along the z-axis.
(ii) Divergence of the x-ray beam in the z-direction results in a cone angle.
(iii) Example: a 64 detector array of 0.625 mm width measures 40 mm along z, requiring a similar beam width obtained by opening the collimator slit at the x-ray tube port.
(iv) Systems allow detectors to bin data from adjacent detector arrays to increase slice thickness.
(v) Larger slice thickness: less image noise (both electronic and quantum), less storage required.
(vi) Larger slice thickness: more partial volume artifact, less z-axis resolution.
(vii) MDCT reconstructed slice thickness and collimated x-ray beam width are separate parameters.
(viii) MDCT allows acquisition of faster scans (whole body in seconds for large width arrays). (ix) MDCT allows thinner slice acquisitions (thinnest slices are sometimes termed “raw” data).
2. Wide-beam (cone-beam) CT systems
a. All whole-body scanners with ≥64 detector channels are technically cone-beam CT systems.
b. True cone-beam scanners: where the half cone angle approaches 9°.
c. Reconstruction algorithms for synthesizing data must take into account the cone angle.
d. Challenges and benefits of wide cone-angle CT systems.
(i) Challenges: increased x-ray scatter detection and cone-beam artifacts
(ii) Benefits: whole organ imaging without table motion (e.g., head, heart, organ perfusion; the latter with high temporal resolution)
e. Cone-beam systems with flat-panel detectors (Fig. 10-8).
(i) Full cone angles almost as large as the fan angle are possible.
(ii) Full 2D bank of detectors and flat geometry, with different reconstruction geometry.
(iii) Detector calibration includes flat-fielding to correct for inverse square variations and heel effect.
(iv) Parallax error (nonnormal incidence to the detector at wide angles) cannot be corrected.
(v) Detector readout rates limit acquisition speed; pulsed x-ray tube projections limit motion.
(vi) Applications: dental CT, breast CT, orthopedic, angiographic, and radiation therapy.
3. The slip ring
a. The CT gantry x-ray tube and detector array rotate continuously in one direction with a slip ring.
b. The slip ring has a number of electrically conductive tracks and associated contactors (Fig. 10-9).
(i) This maintains electrical contact with the stationary frame to the rotating gantry.
(ii) The x-ray transformer is mounted on the gantry to avoid high voltage and electrical arcing.
(iii) Numerous small conductive tracks with low electronic noise transfer digital signal data out.
c. Helical and high-speed rotations are enabled, with high centrifugal forces exceeding 20 g’s.
(i) Special design considerations and hardware are required for all components on the gantry.
(ii) X-ray tube focal spots have flat emitter design with magnetic/electrostatic focusing coils to withstand high gyroscopic forces (see Chapter 6 for details).
4. The patient table (couch)
a. The table is an important and highly integrated CT scanner component.
b. The CT computer controls longitudinal table motion with precision motors and feedback.
(i) Precise control and positioning of the table is important, particularly for helical acquisition.
(ii) Accuracy of anatomic positioning is essential.
(iii) Table position accuracy is evaluated during routine CT scanner testing.
c. Height adjustment allows for convenient patient access and centering in the FOV (Figs. 10-10 and 10-11).
5. The x-ray tube and CT detector characteristics
a. CT x-ray tubes have the highest power loading and heat dissipation ratings.
(i) MDCT acquisition geometry: better use of tube output with larger collimator and beam width.
(ii) Plane of anode rotation is parallel to plane of gantry rotation to reduce gyroscopic forces.
(iii) Anode-cathode axis is parallel to the z-axis of the scanner—so is the heel effect (Fig. 10-12).
(iv) The x-ray output is limited angularly in the anode-cathode direction, but not in the fan angle (Fig. 10-13).
b. Continuous x-ray output is used by most CT scanner manufacturers.
(i) Detector sampling time is the acquisition time for each CT projection acquired.
(ii) Sampling dwell times: approximately 0.2 to 0.5 ms.
(iii) Approximately 1,000 to 3,000 projections are acquired for a 0.5 s gantry rotation over 360°.
(iv) At the periphery of the FOV, the angular displacement of the focal spot during the sampling dwell time is greater than at the center, causing a relatively greater sampling distance, and resulting in blurring and loss of spatial resolution.
(v) Compensation (some CT scanners) steers the focal spot in the opposite direction (Fig. 10-14).
(vi) Some CT scanner manufacturers use focal spot steering to “oversample” the z-dimension to cause a shift in the source position—this effectively doubles the unique angular projection data sampling per rotation, relative to the number of detector channels.
c. Pulsed x-ray output by some manufacturers is used to switch x-ray voltage for dual-energy CT.
(i) Every other pulse is a high (e.g., 140 kV) or low (e.g., 80 kV) projection (see Section 10.3.8).
d. Wider x-ray beam collimation with MDCT increases the detected scatter fraction.
(i) 2D antiscatter grids are used to reduce scatter (Fig. 10-15).
e. Indirect detection (scintillator) solid-state detectors are most widely used (Fig. 10-16).
(i) Scintillator materials are sintered to increase density and improve detection efficiency.
(ii) Ceramic phosphors are scored to produce individual detector elements.
(iii) Opaque filler between detector elements is added to reduce cross-talk.
(iv) Each ceramic detector element has a contact photodiode to generate charge from light.
(v) The detectors are designed in modules with electronics to acquire the digital data.
f. Emerging photon-counting detectors use solid-state detector technology.
(i) Energy discrimination allows individual photons to be assigned a discrete energy bin.
(ii) Multispectral imaging is possible without multiple scans at different tube potentials.
(iii) Technical challenge is the bandwidth of the electronics to measure high photon flux.
6. Over beaming and geometrical efficiency
a. Overbeaming results from the presence of penumbra due to the finite focal spot size (Fig. 10-17).
b. Collimation extends beyond the active detector array configuration to ensure uniform beam.
(i) Active detectors in the penumbra region will generate a skewed slice sensitivity profile.
(ii) Manufacturers must increase the beam collimation beyond the outer active detectors.
c. Beam geometrical efficiency is a function of the active detector width to beam width (Fig. 10-18).
(i) The penumbra width remains constant, independent of beam collimation.
(ii) For wider active detector arrays, efficiency is improved.
(iii) Smaller active detector widths (e.g., 8 channels of a 64 channel array) have poorer efficiency.
(iv) Dose due to overbeaming can be a large fraction of the total dose when a small number of detector arrays are active—low-dose efficiency triggers a warning on the CT scanner.
7. Adapting data acquisition to patient anatomy: noise propagation in CT images
a. Noise in a pixel is the consequence of all projection data intersecting the voxel representing the pixel.
b. Noise variance (Σ2) within the pixel results from the propagation of noise variance of the projections:

c. “Noise adding in quadrature” means larger subcomponents of noise contribute more to the total noise.
(i) Total noise (the standard deviation) is the square root of σ2CT image.
d. Methods to reduce noise are focused on the components of the total noise that have greater impact.
(i) Bowtie filters modify the incident beam to achieve a more uniform x-ray transmission from the center to the periphery of the patient.
(ii) Tube current modulation varies the incident radiation as the x-ray tube rotates around the patient and adjusts current (x-ray output) on the attenuation path for each projection.
(iii) These issues are discussed next and later in the guide.
8. Beam shaping filters
a. Most body parts are circular or approximately circular in shape.
b. Transmission of x-rays through the patient head or torso is greater in the periphery of the projection.
c. The bowtie filter shapes the incident radiation fluence to adjust for the attenuation variability presented by the patient (Fig. 10-19).
d. Reducing the beam intensity peripherally reduces dose to the patient with no appreciable loss of quality.
e. In the absence of a bowtie filter, the dose to the periphery is increased (Fig. 10-20A).
f. With the wrong bowtie filter mismatched to patient anatomy, dose increases centrally (Fig. 10-20C).
9. View sampling
a. The data acquisition system (DAS) is intrinsic to the view sampling rate and sampling size.
b. The rotation period of the gantry about 360° occurs with continuous radiation output.
(i) View sampling rate (frequency) over the rotation period determines sampling size (Fig. 10-21).
(ii) A rate of 2,000 to 3,000 samples/s per detector element is necessary to maintain resolution.
(i) Spatial resolution decreases from the center to the edge of the FOV.
d. Focal spot dithering (Fig. 10-14) can help mitigate the loss of peripheral detail.
10. High-resolution CT scanners
a. Specialty, small FOV scanners have been developed for years (small animal, specimen scanners).
(i) “Micro-CT” can deliver voxel dimensions on the order of 10 µm (but 30 minute scan time…).
b. Cone-beam CT scanners have high resolution because of small detector elements in the flat panel.
c. Whole-body CT scanners are now available to provide high spatial resolution in humans.
(i) One vendor provides 0.25 mm detector widths and 0.25 × 0.25 mm in-plane sampling.
(ii) Focal spots can limit spatial resolution, so six focal spots are provided from very small to large.
(iii) Reconstructed image matrix sizes for these scanners are 1,024 × 1,024 and 2,048 × 2,048.
d. Image noise is a challenge at high resolution—innovative reconstruction algorithms using artificial intelligence and deep learning algorithms may be a solution.
e. High-resolution CT may improve CT diagnostic accuracy across an array of clinical applications.
10.3 ACQUISITION MODES
1. The CT scan radiograph
a. X-ray tube and detector array are stationary—table moves through gantry with x-rays on.
(i) Scanned projection radiograph localizer also known as “scout” “topogram” “scanogram” …etc.
(ii) Technique factors use variable kV (often the same as the axial acquisitions) and low mA.
b. X-ray tube position at 0° (top), 90° (right), 180° (bottom), or 270° (left).
(i) Depending on patient position (supine/prone), anterior/posterior (AP) or posterior/anterior (PA) projections are acquired at 0° or 180°, and lateral projections at 90° or 270°.
(ii) Acquisition of both PA or AP and LAT localizer radiographs are common practice.
(iii) Lateral projection identifies positioning of the patient with the isocenter of rotation (table height).
(iv) PA acquisition is preferred to keep breast dose lower than AP acquisition for female patients.
c. The localizer defines anatomy slightly beyond the beginning and end of the planned CT acquisition.
(i) Using CT console software, the technologist plans the CT scan locations (Fig. 10-22).
2. Axial (sequential) acquisition
a. Basic “step and shoot” where the tube and detector rotate around stationary patient/table (Fig. 10-23).
b. X-rays turn on for 360° to acquire raw data for a number of CT slices, then turn off.
c. Table is then moved with beam off to the next position and axial scan is repeated with stationary table.
d. Between each acquisition, the table moves a distance D, equal to the sampling distance at isocenter.
(i) For contiguous data acquisition D = nT, resulting in contiguous CT images along the z-direction
(ii) n = number of array channels and T = detector width; nT = collimated beam width + overbeaming
e. In some protocols, nT < D, which provides better sampling in z (e.g., for 3D volume rendering), but increases dose if the same technique factors are used.
3. Helical (spiral) acquisition
a. Table moves simultaneous to tube and detector rotation, creating a helical path (Fig. 10-24).
b. X-rays are on constantly; eliminating table start/stop—removes intertial constraints.
c. The pitch describes the relative advance of the CT table per 360° rotation

where Ftable is the table feed distance per 360°
Example: 40 mm = nT and 360° rotation time = 0.5 s; pitch = 1 occurs when Ftable = 80 mm/s
d. Pitch = 1 corresponds to contiguous axial scanning in principle.
e. Pitch >1 represents underscanning of some regions of the body.
(i) Allows for faster scanning (shorter total scan time) to reduce patient motion (e.g., pediatrics)
(ii) For technique factors held constant (except pitch), then

f. Pitch < 1 represents overscanning of the same regions of the body (and higher dose).
(i) Useful for multiplanar reformatting (sagittal, coronal) and 3D volume rendering.
(ii) Often, the technique factors (mAs) are reduced such that the dose is kept constant.
g. Comparison of axial, low pitch helical, and high pitch helical coverage (Fig. 10-25).
h. Acquired data at the beginning and end of scans do not have sufficient angular coverage to reconstruct artifact-free CT images (Fig. 10-26A), requiring over-Useful for multiplanar reformatting ranging.
(i) Results in wasted dose when the x-ray tube is on, equal to ½ nT at beginning and ½ nT at end
i. Adaptive beam collimation on some CT scanners eliminates the overranging penalty (Fig. 10-26B).

▪ FIGURE 10-25 The trajectory of the x-ray source around the patient is shown for (A) axial (sequential) imaging, (B) low pitch helical (spiral) imaging, and (C) high pitch helical imaging. F10-28
F10-28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access