CHAPTER 18
Congenital Cardiac Masses
Tina M. Bachman and Heidi S. Barrett
Definition
Congenital cardiac tumors are rare. The occurrence rate in infants and children is approximately 0.027%.1 Among the pediatric population, more than 90% of cardiac neoplasms are histologically benign. However, they have the potential for serious consequences if not detected in a timely manner, usually because of their conspicuous location.1–4 The incidence of cardiac tumors in the fetus is approximately 0.14%.4
Rhabdomyoma is the most common tumor of both infancy and childhood, occurring in 60% of cases. Teratoma (25%), fibroma (12%), and hemangiomas (3%) occur less frequently.5 Myxoma and neurofibroma have also been reported in childhood but are more commonly found in adults.6–9
Rhabdomyoma
Rhabdomyomas have been diagnosed in the fetus as early as 20 weeks’ gestation.4,10–20 They tend to be multiple (90%), occurring most frequently within the right or left ventricle or within the interventricular septum. They may occur within the myocardium, leading to a variety of arrhythmias (usually supraventricular tachycardia) or may grow within the cardiac chambers, which can lead to valvular obstruction.11,21–26 Most congenital cardiac tumors are isolated anomalies; however, tuberous sclerosis has been reported in 60% to 80% of patients diagnosed with rhabdomyoma.4,10,27 Tuberous sclerosis is a multisystem disease classically characterized by the triad of adenoma sebaceum, epilepsy, and mental retardation. It is a familial disease inherited as an autosomal dominant trait with variable penetrance and variable expressivity. Rarely, it may be a sporadic event.28
The clinical and hemodynamic findings of cardiac rhabdomyomas are related to the number, size, and position of the tumors.29 In the fetus, if supraventricular tachycardia occurs, nonimmune hydrops fetalis and polyhydramnios may manifest. Decreased fetal growth has also been associated with rhabdomyoma.11–18,30 Clinically, in addition to serious arrhythmias, severe cyanosis, mental retardation, seizures, cutaneous lesions, and visceral tumors may be observed in the neonate or infant.9,24,26,31–33
Spontaneous regression of cardiac rhabdomyomas in early childhood occurs in more than 80% of cases.4,34 Although spontaneous regression has been observed, sudden death is not uncommon.10,12,23–26,35,36
Teratoma
Cardiac teratoma is the second most common tumor in the fetus; it accounts for up to 21% of benign cardiac tumors in infants and 14% of such tumors in children aged 1 to 15 years.4,18,37 Intrapericardial teratomas are the second most common tumor reported prenatally. They are usually single, encapsulated tumors attached to the base of the heart.38–42
Intrapericardial teratomas are attached to the heart by a broad pedicle or stalk. Common sites of occurrence include the root of the aorta or pulmonary artery and the right ventricle and right atrium.38–41,43–48 Less frequent locations include the left atrium, left ventricle, and superior vena cava.38,41
The size of intrapericardial teratomas is variable. Asymptomatic older children and adolescents are affected by very small lesions, whereas lesions reported in the neonate or infant have been three to four times the size of the heart.40,41,49
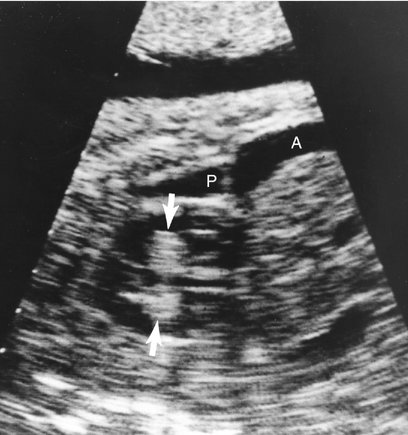
Intrapericardial teratomas in the fetus are almost always accompanied by a pericardial effusion.41,44,46–47 Similar to rhabdomyomas, intrapericardial teratomas are space-occupying lesions that can cause hemodynamic compromise as a result of obstruction and compression of the heart. Sudden death has been reported in 66% of pediatric patients.47 This is thought to result from acute rupture into the pericardial space, causing sudden cardiac tamponade, severe encroachment by the tumor on the heart and great vessels, infectious pericarditis, or a combination of these conditions.38,47,50
Fibroma
Prenatal diagnosis of cardiac fibroma on the basis of sonographic findings is unusual; however, a few cases have been described.51 Cardiac fibromas are usually singular, nonencapsulated intramural lesions that most often involve the left ventricular free wall and interventricular septum.52–60 They are usually located at the left ventricular apex. Other reported locations include the atria, the interatrial septum, and the right ventricular free wall, although this is an uncommon site.52–54,57,61,62
Fibromas may invade the conducting system because of their location, resulting in arrhythmias or sudden death.37 As with all cardiac tumors, sequelae vary depending on the size and location of the tumor. Large fibromas may encroach on the intracavitary space, causing subaortic and subpulmonic obstruction.63 Pedunculated fibromas that obstruct the outflow tracts have also been observed.
Clinical symptoms in the neonate include severe congestive heart failure and cyanosis.52,54,56,63,64 Significant pericardial effusions have also been associated with fibromas.29
Hemangioma
Cardiac hemangiomas are rare, benign vascular tumors that account for 2% to 5% of all cardiac masses.7,37,65–67 They can involve any chamber of the heart and have also been reported to invade the interventricular septum or atrioventricular node, resulting in rhythm disturbances.65,67–69 Although hemangiomas are usually asymptomatic, growth occurring within the epicardium or pericardium may cause pericardial effusion and cardiac tamponade.65–67,69 Prenatally, these polypoid masses may be diagnosed by demonstrating the main feeding vessel with color or power Doppler imaging.70
Myxoma
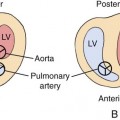
Cardiac myxomas are the most common primary cardiac tumor in adults.3,68,71,72 Although they have been reported in the neonate, they are exceedingly rare.73–77 They have not been reported in the fetus. Myxomas arise from the endocardium, occurring in the left atrium in 75% of patients and in the right atrium in 25%.72,78,79 These tumors are usually singular lesions that are attached to the foramen ovale by a pedicle.29,37 Attachment to the left atrial free wall has also been reported.80
The classic appearance of a cardiac myxoma is that of a large pedunculated tumor traversing back and forth through the atrioventricular valve.71,81–87 Classified as benign, these tumors have a tendency for local recurrence and, rarely, malignant degeneration.88–90
Although more than 90% of myxomas occur sporadically, they have also been seen in children and adolescents with multiple lentigines syndrome.91 These patients have pigmented skin lesions and endocrine neoplasms in addition to the cardiac tumor.37
Myxomas present with a classic triad of symptoms that includes hemodynamic obstruction, emboli, and systemic illness.71,72,79,92,93 Rarely are patients asymptomatic. Most pediatric patients (80%) have symptoms of valvular obstruction.87
Large left-sided myxomas have been reported to obstruct pulmonary venous inflow, resulting in pulmonary edema, pulmonary arterial hypertension, and low cardiac output.71,72,90 They may also cause left ventricular inflow obstruction and mimic mitral stenosis. Right atrial tumors impede systemic venous inflow and cause right ventricular failure and low cardiac output.71–75 Myxomas may mimic neonatal cyanotic heart disease when an obstructive right-sided tumor causes right-to-left shunting at the atrial level.73–76 Myxomas have also been associated with sudden death.73,74
Congenital Ventricular Diverticulum and Aneurysm
Other masses that occur in the fetal heart include congenital ventricular diverticulum (CVD) and congenital ventricular aneurysm (CVA). The terms CVD and CVA have been used in the literature interchangeably. However, they are two distinct entities with different histological and morphological characteristics.94 Their true incidence is unknown because most cases are asymptomatic, but the prevalence of CVA has been suggested to be 0.5 in 100,000 births.95
The two entities have been variably classified according to four major criteria. These criteria include connection to the ventricular cavity, wall composition, wall motion, and association with intracardiac abnormalities or midline defects.94–97 CVDs have a narrow connection to the ventricular cavity, whereas CVAs have a wide neck or broad connection to the ventricular cavity.94–97 Histologically, CVDs are composed of the normal layers of the ventricular wall. Comparatively, CVAs tend to be fibrous with thinned myocardium or an outpouching through disrupted myocardium.95,96 Because of the histological nature of these two entities, a CVD will contract during systole with the associated ventricle, whereas a CVA does not. This has been recognized as the most accurate parameter for diagnosis by ultrasonography.94–96 The final classification is an association with intracardiac abnormalities or midline defects. Both CVDs and CVAs are usually isolated lesions; however, some associated cardiac anomalies have been reported with CVD.98 Marijon et al92 evaluated 16 cases of CVD. They identified two subgroups that were distinguished by location of the CVD in the ventricle. The apical CVD was a fingerlike contractile pouch with a narrow connection to the ventricle that was consistently associated with midline thoracoabdominal defects. Apical CVDs were always associated with cardiac rotation disorder or intracardiac abnormalities. In contrast, nonapical CVDs were described as a contractile pouch with a wider connection to the ventricle and were not associated with other abnormalities.94
Cardiac diverticula and aneurysms are thought to occur as a result of a focal weakening of the ventricular wall because of an interruption during embryogenesis, infection, or localized ischemia.98
Pseudomasses
A persistent or redundant eustachian valve can mimic an atrial mass.99,100 The eustachian valve originates at the junction of the right atrium and the inferior vena cava.99 It diverts blood flow from the inferior vena cava into two separate channels, allowing oxygenated blood from the ductus venosus to flow through the foramen ovale into the left atrium. Deoxygenated blood entering the inferior vena cava is directed through the tricuspid valve into the right ventricle.99,100 In the first trimester, the eustachian valve is large, covering the mouth of the inferior and superior vena cavae.99 As pregnancy progresses, the eustachian valve decreases in size and may disappear. It may also persist into adulthood. A wispy or circular structure should be recognized as a normal eustachian valve.
Echogenic intracardiac foci are small discrete structures found within the cardiac ventricles in the region of the papillary muscles or chordae tendinae. They are described as having echogenicity comparable to that of fetal bone and by autopsy correlated with areas of mineralization. They occur in the left ventricle 90% of the time but may also be identified in the right ventricle or bilaterally.101–103 Echogenic foci are common in normal fetuses, occurring in 3% to 5% of the normal population.102
Echogenic foci should not be considered a cardiac mass. They are not associated with structural heart disease, nor do they cause any hemodynamic disturbance.102,104 Previously, echogenic foci had been associated with chromosomal abnormalities such as Down syndrome.102 More recent literature has cast doubt on this association.101–105
Sonographic Criteria
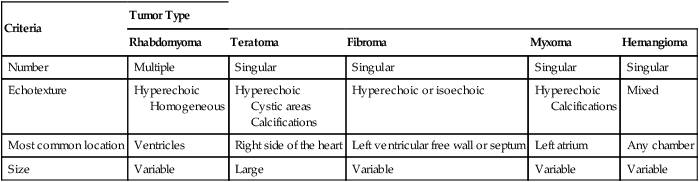
Most fetal cardiac masses are easily recognized on ultrasonography. The prenatal diagnosis of a cardiac tumor is usually an incidental finding on routine obstetrical ultrasonography.106 The presence of an intracardiac mass in the fetus statistically suggests rhabdomyoma. Although it is impossible to make a histological diagnosis in utero, several sonographic criteria, including tumor number, size, location, and echogenicity, may help narrow the differential diagnosis (Table 18–1). The presence of multiple tumors is indicative of rhabdomyoma.* Sonographically, they appear as hyperechoic, heterogeneous masses of variable size. They are unencapsulated, well-circumscribed lesions.10–18 Areas of fibrosis or calcification have been identified within them.108
TABLE 18–1
Sonographic Criteria of Cardiac Tumors
| Criteria | Tumor Type | ||||
| Rhabdomyoma | Teratoma | Fibroma | Myxoma | Hemangioma | |
| Number | Multiple | Singular | Singular | Singular | Singular |
| Echotexture | Hyperechoic Homogeneous | Hyperechoic Cystic areas Calcifications | Hyperechoic or isoechoic | Hyperechoic Calcifications | Mixed |
| Most common location | Ventricles | Right side of the heart | Left ventricular free wall or septum | Left atrium | Any chamber |
| Size | Variable | Large | Variable | Variable | Variable |
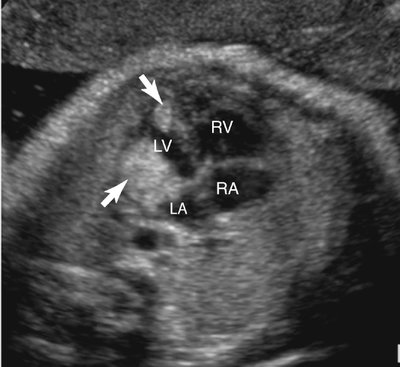
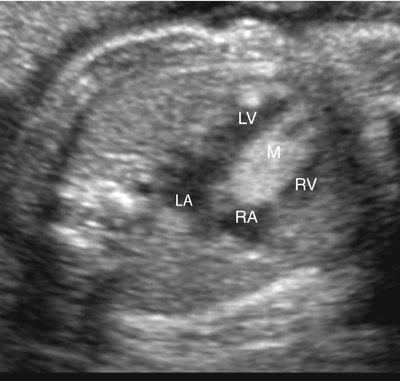
Rhabdomyomas may occur anywhere in the heart but are most often located in the ventricular myocardium (Fig. 18–1).4,109 Frequently the tumor appears to involve both sides of the interventricular septum.59 Intracavitary extension is found in up to 50% of cases.4 Rhabdomyomas may simulate a hypoplastic heart if the tumor becomes large enough to obliterate the ventricle (Fig. 18–2).
Rhabdomyomas obstructing the mitral or tricuspid valves have been reported to simulate atresia of the respective valve (Fig. 18–3).1,23,26,110–120 They often obstruct valvular inflow or outflow, or both; therefore Doppler imaging of the valve or valves located near the mass is important.
Single pedunculated rhabdomyomas have been associated with subaortic stenosis and pulmonic valve stenosis.*
Because of the strong association of rhabdomyoma with tuberous sclerosis, the fetus should be evaluated for other stigmata of this syndrome, including tumors of the brain, liver, or kidneys and renal cysts.10,123–127
A family history of tuberous sclerosis or evidence of other organ system involvement is fairly indicative of rhabdomyoma. However, a negative family history should not preclude the diagnosis because spontaneous mutations do occur.29
The majority of rhabdomyomas may significantly decrease in size or disappear toward the end of the third trimester, whereas other tumor types should not.4,29 However, despite this decrease in size, fetal loss may occur as a result of arrhythmia or congestive heart failure.4
Teratomas are usually singular lesions that appear heterogeneous, lobulated, and encapsulated on ultrasonography.38,39,43–46,48,128 They may contain cystic areas or echogenic foci.43,45–48 They are usually detected in the pericardial cavity attached to the aorta or pulmonary artery.4 Doppler interrogation is necessary to evaluate the hemodynamic consequences of the tumor. Tumor extension into the superior vena cava has also been reported.38 A stalk or pedicle may be seen attaching the tumor to the heart.39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree