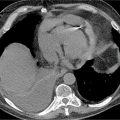
FIGURE 9-1 Frame from a horizontal long-axis SSFP cine acquisition demonstrates enlargement of the RA and RV.
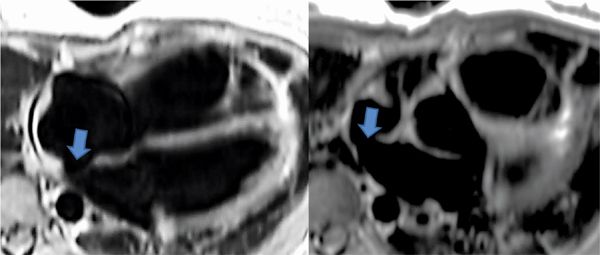
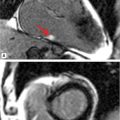
FIGURE 9-2 Dark-blood double inversion recovery (DIR) imaging in the axial plane at the AV valve level (left) and at SVC-RA junction (right) suggests the presence of a superior sinus venosus ASD (arrows).
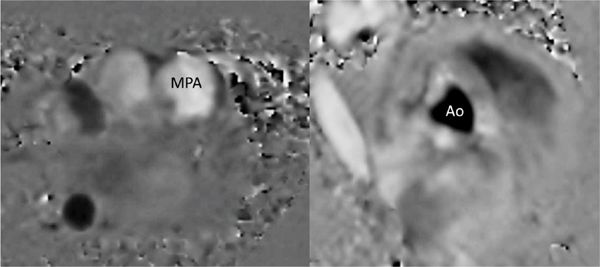
FIGURE 9-3 Through-plane VENC cine acquisitions are prescribed across the left MPA, and right aorta to allow quantitation of pulmonary flow (Qp) and systemic flow (Qs), from which the Qp/Qs ratio can be computed.
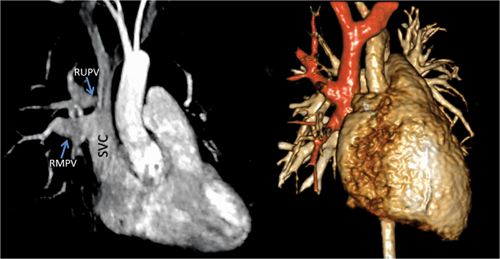
FIGURE 9-4 Contrast-enhanced MRA (CE-MRA) (left) demonstrates the presence of a partial anomalous pulmonary venous return of the rUpV and RMPV to the SVC. the same anatomy is shown in a volume rendering (right) with the SVC, anomalous pulmonary veins (PVS) and sVC tributaries highlighted in orange.
DISCUSSION
Atrial septal defects are the third most common type of CHD with an estimated incidence of 1 per 1500 live births.8,9 The secundum ASD is the most common type with a defect of the septum primum within the fossa ovale. A primum ASD is typically associated with the endocardial cushion and is a variant of an incomplete common atrioventricular (AV) canal defect and is the second most common type of ASDs. The third most common ASD is the superior sinus venosus ASD which results in deficiency of the superior rim of the sinus venosus septum which separates the pulmonary and systemic veins and the sinus venosus component of the RA.10 As a result there is a very high association of right-sided pulmonary vein drainage abnormalities. A small to moderate isolated sinus venosus ASD by itself may not result in significant volume load on the right heart; however, that in combination with PAPVR can lead to significant volume load on the right heart as seen in this patient. In untreated patients with ASD, long-term exposure to increased pulmonary blood flow can lead to pulmonary vascular disease in 5% to 10% of patients, typically after age 20 years.
Although TTE demonstrated right-sided chamber enlargement, the etiology could not be elucidated due to poor acoustic windows. CMR was able to overcome the limitations of TTE by demonstrating not only the small to moderate superior sinus venosus ASD but also associated PAPVR of the RUPV and RMPV. In addition, the PC quantitation demonstrated a Qp/Qs ratio of 1.9 to 1, indicating significant volume load on the right heart with increased risk of pulmonary vascular disease (PVD). Since reports show that adults with ASD with Qp/Qs ratio of greater than 1.5 to 1 not only are at risk of developing PVD but die at an earlier age, she was scheduled for surgical repair of her defects.11 In this case the findings from the CMR resulted in surgical intervention to prevent future risk of arrhythmia, PVD, and premature death.11,12
CASE 2 Ventricular Septal Defect
A 15-year-old girl with a known ventricular septal defect (VSD) diagnosed in infancy presented for routine clinical evaluation. Her history was unremarkable for cardiovascular symptoms. However, on cardiac examination she was noted to have a new murmur in addition to the holosystolic VSD murmur. She underwent routine TTE examination that demonstrated presence of the VSD as well as RV muscle bundles resulting in creation of a double chamber right ventricle (DCRV) leading to severe right ventricular outflow tract obstruction (RVOTO). However, the severity of the RVOTO was nonconsistent with her physical examination findings and there was no evidence of significant right ventricular hypertrophy (RVH) by TTE. Therefore, CMR was requested and performed to clarify the exact anatomy of the RVOT as well as to assess cardiac chamber sizes, ventricular mass and systolic function.
Pertinent sequences included SSFP cine in horizontal long-axis (HLA) and short-axis planes for ventricular size and function, DIR, and SSFP cine stacks through the RV outflow. The HLA acquisition demonstrated normal chamber sizes with no significant RVH (Figure 9-5). A small perimembranous VSD can be seen in a more superior slice (Figure 9-6A) as well as in a short-axis view at the base of the heart (Figure 9-6B). The RV muscle bundles can be seen in an RVOT SSFP view leading to a DCRV and subsequent dynamic RVOT obstruction (Figure 9-7).
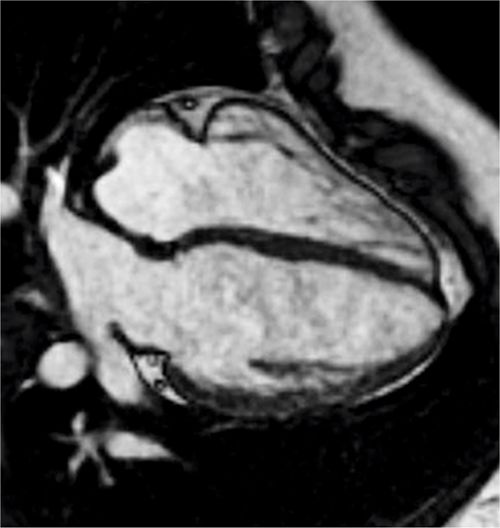
FIGURE 9-5 A frame from a horizontal long-axis SSFP acquisition at the level of the AV valves demonstrates normal chamber sizes with no significant RVH in a patient with a history of VSD and suspected RVOTO by TTE.
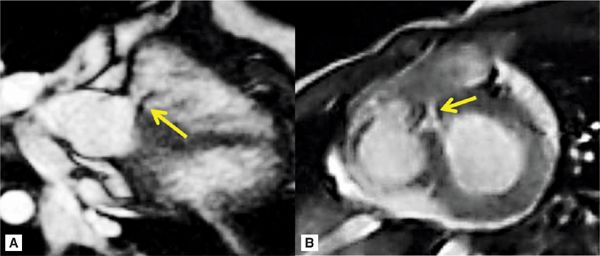
FIGURE 9-6 A small perimembranous VSD (arrows) can be seen in a more superior horizontal long-axis SSFP acquisition (left) as well as a short-axis view at the base of the heart (right).
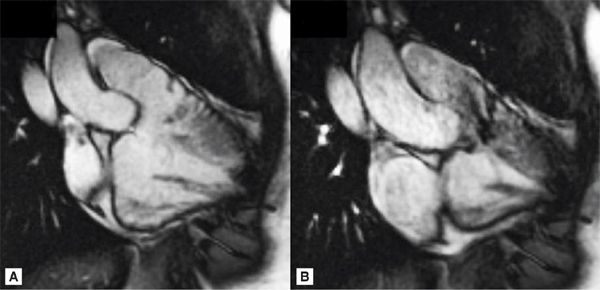
FIGURE 9-7 Diastolic (left) and systolic (right) frames from a RVOT SSFP view show RV muscle bundles leading to a DCRV and subsequent dynamic RVOT obstruction.
DISCUSSION
Ventricular septal defect is the second most common type of CHD and comprised 20% of CHD with an incidence range of 2 to 50 cases per 1000 live births depending on which literature is cited.8 The prevalence of VSD is in older children and is lower in adults due to spontaneous closure in some patients.13 Ventricular septal defects are classified based on their location on the ventricular septum. Perimembranous VSD is the most common and comprised approximately 80% of surgical and autopsy series. Outlet or supracristal VSD is less common in the West and comprises 5% to 7%, but is more common in the Far East where it comprises almost 29% of all VSDs. Inlet VSD (5%-8%) is typically associated with endocardial cushion defects. The true incidence of muscular VSD is unknown but is estimated to comprise 5% to 20% of all VSDs.14–16 Large defects are typically repaired within the first year of life.17 Patients with unrepaired large VSD will develop PVD with increasing risk through adulthood and eventual Eisenmenger syndrome.18–20 Small to moderate isolated VSD typically will not require surgery and can spontaneously close but should be monitored for development of aortic valve prolapse, aortic ridge, and DCRV.21–23
Although TTE demonstrated significant RV muscle bundles with DCRV and resultant severe RVOTO, CMR was able to confirm no evidence of RVH and that the RVOTO was only mild to moderate. Although CMR is not routinely indicated for evaluation of isolated VSD, it is a useful alternative to invasive TEE or cardiac catheterization when TTE is inadequate.24,25 The findings of only mild to moderate dynamic RVOTO with no significant RVH on CMR imaging impacted the patient’s medical care significantly by altering the decision to monitor the patient rather than move forward with surgical repair. Due to continued findings of severe RVOTO by TTE, the patient underwent a cardiac catheterization 4 years later which confirmed that there was only mild to moderate RVOTO as demonstrated by CMR. The patient continues to be asymptomatic and clinically doing well.
CASE 3 Atrioventricular Septal Defect
A 5-day-old newborn with Down syndrome underwent TTE that showed a transitional atrioventricular (AV) canal defect comprised of a large primum ASD and common AV valve with no VSD component. The patient was also noted to have aortic arch hypoplasia and a large patent ductus arteriosus (PDA) as well as a left ventricle (LV) that was not quite apex forming. Although TTE was helpful in identifying the major abnormalities, the unanswered question was whether he could undergo a 2-ventricle repair vs a single-ventricle palliation. Therefore, CMR examination was performed to confirm the anatomy but in particular to evaluate the aortic arch and provide precise right and left ventricular volumes to determine whether the LV was adequate for a 2-ventricular repair.
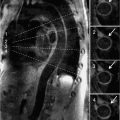
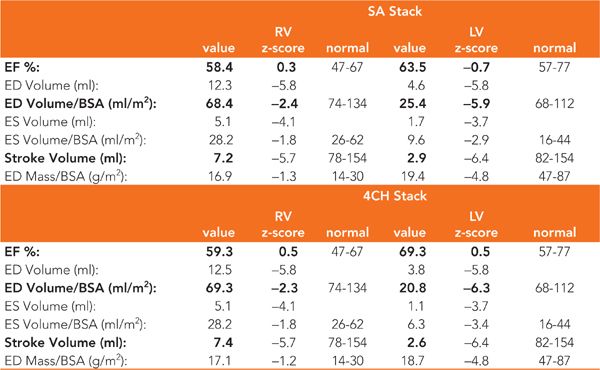
CMR examination was performed in a 1.5 T magnet with the patient under general anesthesia. Pertinent sequences included SSFP cine and DIR imaging in the HLA and SAX planes for ventricular size and AV canal assessment. An MRA was performed to further detail the aortic arch and PDA anatomy. Imaging in the HLA plane demonstrated a large primum ASD with an intact inlet ventricular septum as well as the hypoplastic nonapex forming LV (Figure 9-8). Contiguous HLA or 4-chamber (4-CH) cines as well as a contiguous stack of short-axis SSFP cine acquisitions were used for ejection fraction (EF) and ventricular volume calculation yielding similar results (Table 9-1). Ventricular function by EF was normal, with LV volumes less than half those of the RV though still sufficient for biventricular repair.26 Volume-rendered reconstructions of the MRA demonstrated mild aortic valve and transverse aortic arch hypoplasia and a large PDA (Figure 9-9).
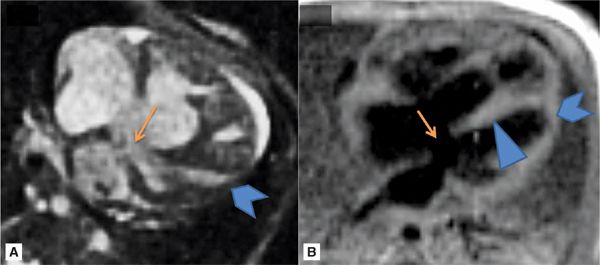
FIGURE 9-8 HLA SSFP frame (A) and DIR image (B) demonstrate the large primum ASD (arrows) with an intact inlet ventricular septum (arrowhead) as well as the hypoplastic nonapex forming LV (chevrons).
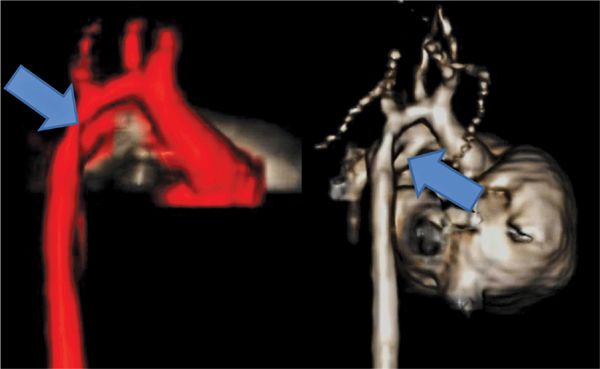
FIGURE 9-9 Volume-rendered reconstructions of an MRA in a 5-day-old newborn with Down syndrome (Case 3) demonstrate aortic arch hypoplasia (left, arrow) and a large PDA (right, arrow).
TABLE 9-1 sVolumetric calculations from contiguous short-axis (SA) SSFP cines as well as a stack of horizontal long-axis or 4-chamber (4CH) cines are similar in this patient with AV canal defect. Also note that left ventricular (LV) volumes are less than half of right ventricular (RV) volumes.
DISCUSSION
Atrioventricular septal defect as a group accounts for only 4% to 5% of CHD and has an estimated incidence of 0.2 per 1000 live births.27–30 Approximately 45% of children with Down syndrome have CHD with more than 40% of these patients having a variant of AVSD.27 Classification of the type of AVSD is based on the relationship of the AV valves and the VSD. Patients with complete-balance AVSD will act like VSD and ASD and require earlier surgical repair. Patients with transitional or partial AVSD will have discordance of the AV valve morphology, and the VSD size renders physiology similar to those of patients with ASD.31,32 Association of unbalanced ventricle and arch obstruction complicates the timing and type of surgery the patient will require.33–35 The most important decision in the neonatal period is whether the patient can under go a biventricular repair, which must be done early due to risk of developing PVD earlier.36–38
Although the LV and the AA were both hypoplastic, the association of Down syndrome made the single pathway more risky due to concerns of pulmonary hypoplasia and high risk of PVD in these patients.34 In addition, CMR findings of LV volume greater than 20 mL/m2—a controversial cut-off—supported that biventricular repair was feasible.39–41 Thus the patient underwent complete repair with ASD closure and aortic arch repair. Postoperative TTE has shown some growth of his LV.
CASE 4 Bicuspid Aortic Valve
A 15-year-old male athlete presented to the pediatric cardiology clinic with a nonspecific chest pain that was reproducible by palpation. His vital signs and physical examination were normal except for a systolic ejection click. He subsequently underwent TTE, which demonstrated a bicuspid aortic valve (BAV) with significant aortic root dilation. Due to the size of the aorta, CMR was requested to further assess the aortic valve, aortic root and the remainder of the aorta which could not be evaluated by TTE.
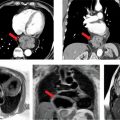
Pertinent sequences included SSFP cines, PC of the aortic valve, DIR stacks, and an MRA. SSFP in the 3-CH view demonstrated significant dilatation of the aortic root with effacement of the ST junction (Figure 9-10). Magnitude and PC images from a VENC cine acquisition across the aortic valve (Figure 9-11) demonstrate BAV and typical nontriangular appearance of the orifice during systole; quantitation demonstrated no significant aortic stenosis (AS) or regurgitation. Sagittal DIR and volume rendering of MRA (Figure 9-12) demonstrated aneurysmal enlargement of the aortic root, ST junction and AA, with a maximum diameter of 4.8 cm. Although there was significant dilatation, there was no evidence of dissection. The arch and descending aorta were normal size.
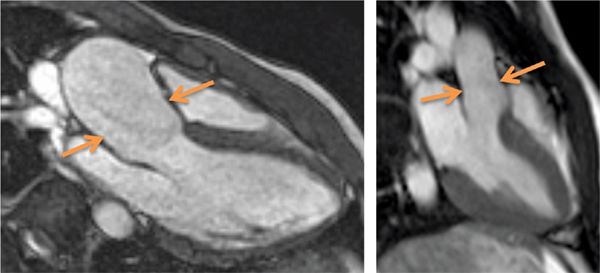
FIGURE 9-10 An SSFP frame in a 3-CH plane shows aortic root dilatation with effacement or loss of the normal shape of the sino-tubular junction (left panel, arrows) in this patient with bicuspid aortic valve. Normal appearance of the sino-tubular junction is shown in the right panel for comparison.
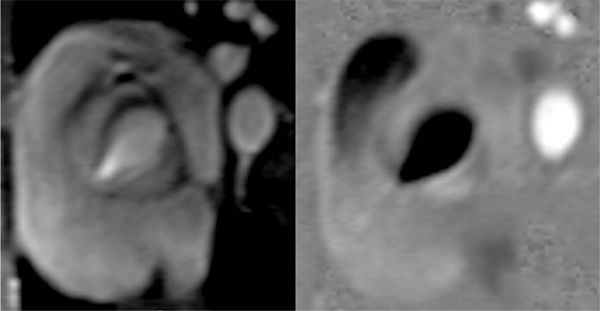
FIGURE 9-11 Magnitude (left) and phase-contrast (PC) (right) images show a bicuspid aortic valve.
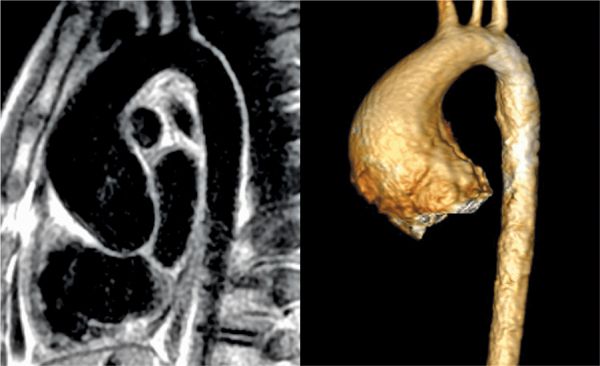
FIGURE 9-12 Double inversion recovery (DIR) image acquired in the sagittal plane (left) and volume rendering of CE-MRA (right) show diffuse dilatation of the aortic root, sino-tubular junction, and AA in this patient with a bicuspid aortic valve.
DISCUSSION
Bicuspid aortic valve is the most common CHD and comprises 1% to 2% of all individuals on autopsy series.42 In some series at least 33% of patients with BAV have complications of aortic stenosis, aortic insufficiency (AI), and aortic root dilation with a smaller number of patients with aortic dissection.43 CMR is extremely useful in detailing the valve morphology as well as associated aortic enlargement. The magnitude and PC images from VENC cine acquisitions provide detailed anatomy of the aortic valve while allowing quantification of flow across the aortic valve for both detail morphologic and functional assessment of the aortic valve.44 As a result of the detailed information provided by CMR, the severity and extent of aortopathy associated with BAV can be demonstrated. This patient was started on losartan and was restricted from competitive sports. Since his initial diagnosis, he has had serial CMR examinations demonstrating stable aortic dimensions with medical therapy.
CASE 5 Coarctation of the Aorta
A 13-year-old patient presented for evaluation of a new murmur. Past history included systemic hypertension for 8 months with associated headaches that had been occurring more frequently. Although she had been active, she reported less energy compared to her friends. In the pediatric cardiology clinic, her blood pressure was at the 95th percentile for age. She was noted to have a grade II/VI medium-frequency systolic murmur in the right upper sternal border. She had significant brachio-femoral delay on palpation of pulses with nearly no femoral pulses appreciated. TTE was performed that demonstrated an abnormal aortic arch with a tortuous area of narrowing just distal to the origin of the left common carotid (LCC) resulting in severe short segment aortic arch narrowing. She was referred to the cardiac catheterization team for possible balloon angioplasty and stent placement. However, as a result of multiple echogenic areas within the lumen of the aortic arch by TTE, CMR was requested to better delineate aortic arch prior to transcatheter repair.
CMR was performed on a 3.0 T magnet with DIR imaging in the axial and sagittal planes as well as an MRA acquired in an oblique sagittal slab. SSFP cines (Figure 9-13) demonstrated no significant left ventricular hypertrophy (LVH) with normal left heart chamber sizes, ventricular mass and biventricular systolic function. The axial and sagittal DIR images (Figure 9-14) demonstrated severe coarctation of the distal transverse and proximal descending thoracic aorta which involves the ostium of the left subclavian artery. The narrowing begins just after the left carotid origin and tapers rapidly. There is a beaded appearance with mild focal dilatation towards the distal aspect of the coarctation. The extent of the narrowing and involvement of the ostia of the left subclavian artery can be better appreciated in the volume-rendered reconstruction of the aortic arch (Figure 9-15). There is mild poststenotic dilation of the descending aorta. Although there were echogenic areas seen on TTE, there was no aortic thrombus by CMR. Based on the aortic anatomy delineated by CMR, the patient underwent balloon dilation followed by stent placement without complications and with reduction in blood pressure and headaches.
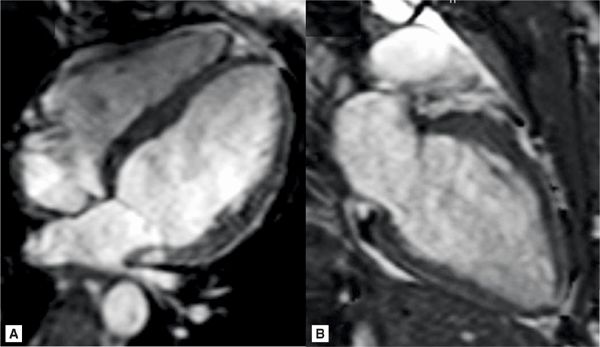
FIGURE 9-13 Horizontal and vertical long-axis cine frames in a 15-year-old with newly-diagnosed coarctation show the absence of LVH.
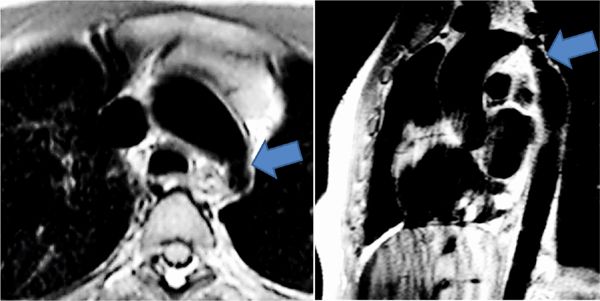
FIGURE 9-14 Axial (left) and sagittal (right) Dir imaging in a 15-year-old with hypertension and brachio-femoral pulse delay show severe coarctation of the distal transverse and proximal descending thoracic aorta (arrows).
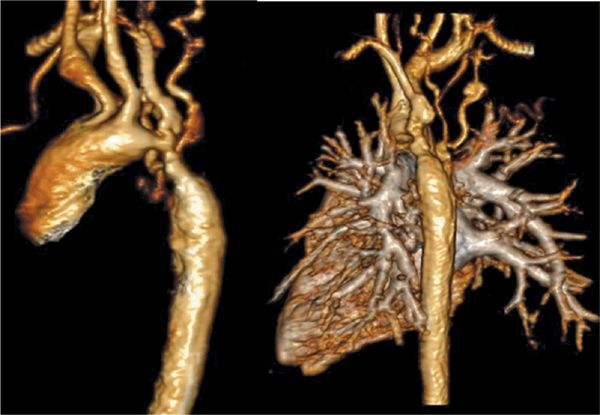
FIGURE 9-15 Volume renderings of the MRA viewed from the left (left) and posterior (right) perspectives demonstrate severe aortic coarctation and mild poststenotic dilatation of the descending thoracic aorta.
DISCUSSION
Coarctation of the aorta accounts for approximately 6% to 8% of all CHD or about 350 cases per 1 million live births with increased incidence in certain syndromes like Turner syndrome in which 35% of patients are affected.27,45,46 Coarctation of the aorta is typically represented by a discrete narrowing of the proximal thoracic aorta but can present with varying degrees of narrowing of the aortic isthmus. Patients can present early in the neonatal period with heart failure or hypotension with a shock-like presentation. Alternatively, like the case being discussed, patients may present later in life with hypertension and decreased lower extremity pulses. In either scenario, it is recommended that patients undergo repair either surgically or by transcatheter balloon angioplasty and stent placement to prevent long-term risk of congestive heart failure (CHF), premature coronary artery disease (CAD), cerebral aneurysm, aortic dissection as well as persistent hypertension.47–51
CMR findings positively contributed to the patient’s medical care by demonstrating no thrombus in the aortic arch as well as the extent of the stenosis. The volume-rendered reconstructions of the aortic arch provided a roadmap for cardiac catheterization which improved efficiency and safety. The patient had successful stent placement with significant reduction in the gradient across the area of narrowing rather than undergoing surgical repair. The initial use of CMR for evaluation of aortic arch abnormalities occurred more than 3 decades ago and continued to be an important cost-effective modality in this disease and has essentially replaced diagnostic cardiac catheterization as an adjunct to TTE for primary diagnosis or follow-up of suspected re-coarctation of the aorta in patients with prior repair or stent placement and provide high-resolution imaging of the aortic wall.52–56
CASE 6 Vascular Ring (Double Aortic Arch)
An 8-month-old infant presented with noisy breathing at rest and sometimes while eating, coughing, and intermittent high-pitched sounds. Multiple chest radiographs during these episodes at a local community hospital found no abnormality. However, approximately 1 month ago, respiratory symptoms increased in frequency and severity. Repeat chest radiography reported an enlarged heart. She was subsequently seen by a local pediatric cardiologist who performed a TTE that was normal, but the patient’s restlessness yielded a suboptimal study. She was referred for further evaluation and underwent ECG-gated CT angiography of the chest.
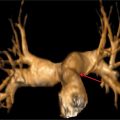
Figure 9-16 shows thin section as well as maximum-intensity projection images reconstructed from the resulting CT data. Axial plane CT image at the level of the double aortic arch shows that it bifurcates into the left aortic arch and slightly larger right aortic arch. An oblique axial maximum-intensity projection reconstruction demonstrates the complete ring formed by the double aortic arch. A thick coronal maximum-intensity projection shows the double aortic arch with mirror-image branching. 3D volume-rendered reconstruction of the double aortic arch viewed from the front, back and top confirms that the right arch is slightly larger (Figure 9-17). Volume-rendered reconstruction of the double aortic arch and airway demonstrates stenosis of the distal trachea due to extrinsic compression by the double aortic arch (Figure 9-18).
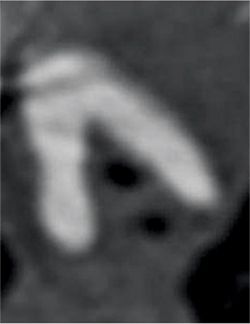
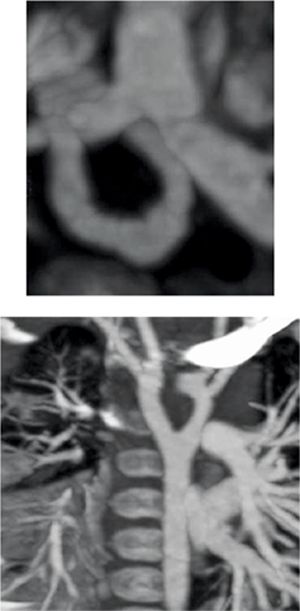
FIGURE 9-16 Axial plane CT image at the level of the double aortic arch (top panel) shows that it bifurcates into the slightly larger right aortic arch and the left aortic arch. An oblique axial maximum-intensity projection reconstruction (center panel) demonstrates the complete ring formed by the double aortic arch. Coronal thick-slab maximumintensity projection image (bottom panel) shows the double aortic arch with anomalous origins of great vessels from both arch segments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree