Congenital Lung Lesions
Teresa Chapman, MD
LEARNING OBJECTIVES
1. Provide a differential diagnosis for a congenital lung mass on prenatal ultrasound.
2. Discuss an appropriate postnatal workup for congenital chest mass lesions, and list two reasons for surgical resection.
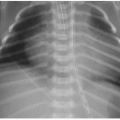
3. Recognize temporal changes in degree of opacification of congenital lobar overinflation (CLO) on chest radiography.
4. Recognize pathophysiologic factors and congenital associations impacting survival with diagnosis of congenital diaphragmatic hernia.
5. Recognize findings on prenatal imaging that indicate favorable prognosis in the context of congenital diaphragmatic hernia.
INTRODUCTION
Developmental anomalies of the lung potentially impact pulmonary development and cardiac function in utero and typically require surgical resection postnatally. Bronchopulmonary foregut malformations, including congenital pulmonary airway malformations (CPAM), bronchopulmonary sequestrations (BPS), and hybrid lesions, are commonly diagnosed by prenatal ultrasound and managed from that point forward.1 In other cases, these anomalies are diagnosed postnatally due to a complication such as infection, respiratory difficulty, or pneumothorax.2,3 Other entities to be considered within this category of disease include CLO (emphysema), which overlaps pathogenetically with bronchial atresia in some cases,4,5 and congenital diaphragmatic hernia (CDH). The latter condition does not arise from the lung but results in a space-occupying lesion, similar to the congenital lung masses mentioned above. In this chapter, the known pathogenesis, imaging, and management options for these developmental anomalies of the lung are discussed.
CONGENITAL PULMONARY AIRWAY MALFORMATION
CPAM is a type of bronchopulmonary foregut malformation that was formerly termed congenital cystic adenomatoid malformation (CCAM). The latter term was replaced by CPAM after a consensus in the pathology literature that an adenomatoid component was not uniformly present. The CPAM can be thought of as a hamartomatous malformation of the tertiary bronchioles, and it has an incidence of approximately 1 in 25,000 to 35,000 live births.1,2 The histology of these masses is quite variable, ranging from solid to multicystic lesions. The histologic classification has been described by Stocker and is based on the developmental origin of the lesion anatomically.6 Three of the five histologically classified lesions can be recognized by imaging: CPAMs with macroscopic cysts (type I contains large cysts; type II contains small cysts) and CPAMs that appear solid (type III, which contain innumerable microscopic cysts or may be truly solid). The CPAM typically communicates with the proximal airways, and most CPAMs receive blood supply from the pulmonary arterial system and drain via the pulmonary venous system. Hybrid lesions, which pathologically have features of both CPAM and BPS (discussed below), may receive blood from the systemic arterial supply.1
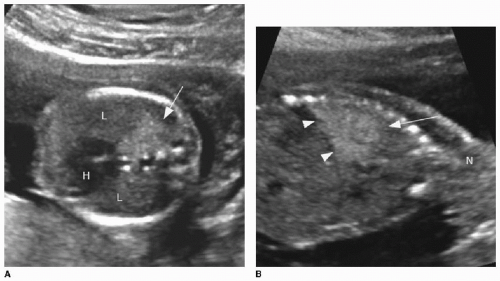
Prenatal ultrasound appearance will be influenced by the size of the mass and by its cystic composition. Solid masses tend to appear homogenous and echogenic. The lesions are typically well demarcated against normal fetal lung (Fig. 4.1). Visualization of the mass on fetal MR may be limited depending on the fluid content of the mass, which may be quite similar to the background lung signal on T2-weighted imaging. Indicators of a poor prognosis include large lesions with mediastinal shift, bilateral lung involvement, and hydrops.7, 8, 9 and 10 Prenatal interventions in life-threatening cases include prenatal steroid therapy, thoracocentesis of dominant cystic components, thoracoamniotic shunting, laser sclerosis, and open fetal surgery.1,11 In up to 65% of cases, these lesions spontaneously regress during gestation.1,3,11
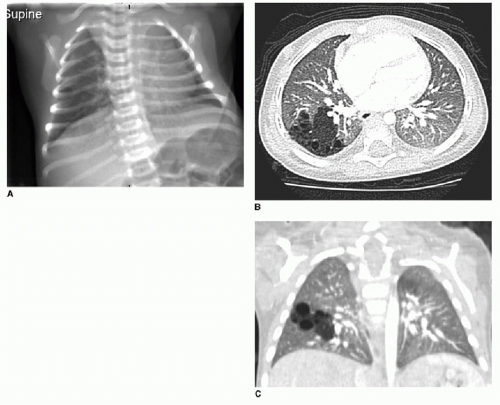
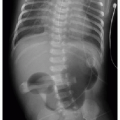
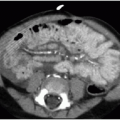
Postnatal chest radiography is often normal,3 possibly due to spontaneous regression of the lesion after prenatal diagnosis. If the lesion is visualized, the radiographic appearance of a CPAM on postnatal radiography will depend on the timing of imaging, as the fluid content of the mass will change. Typically, the mass will gradually become more aerated over days to weeks, as the fetal fluid clears from the cystic components of the mass through abnormal airway connections. Therefore, even a large CPAM with macrocystic components will appear to be a solid mass on chest radiography if the neonate is imaged soon after birth. Later, an intrapulmonary lesion with well-demarcated margins and lucent centers may be appreciable (Fig. 4.2). Cross-sectional imaging with CT is useful in evaluating extent of the congenital abnormality and its relationship to the airways and blood vessels. The CPAM will be evident by multiple variably sized air-filled and possibly cystic spaces, and the overall abnormality may display mass effect upon adjacent lung (Fig. 4.3).
Postnatal management of CPAMs, along with other congenital lung mass lesions, is surgical resection. The timing of surgery is based on symptoms, with prompt excision indicated in patients with significant respiratory distress; however, even the asymptomatic patient with a prenatally diagnosed congenital lung abnormality will undergo surgical resection to avoid the potential for future infection.2,12,13 Another reason to resect cystic lung lesions is to ensure the abnormality is not a pleuropulmonary blastoma, a malignancy that may appear cystic, mixed cystic and solid, or solid and often indistinguishable from a congenital bronchopulmonary malformation.14 Resection of antenatal-diagnosed lesions is typically performed between the neonatal period and approximately 6 months of age. These lesions may be resected by either open thoracotomy or thoracoscopy, although the postoperative course is typically longer following an open approach.12
BRONCHOPULMONARY SEQUESTRATION
BPS is a region of lung parenchyma that does not develop a normal connection to the tracheobronchial tree and also has an anomalous systemic blood supply. The embryologic origin of BPS is thought to be the same as the CPAM,1 and hybrid lesions are found that histologically share features of both lesions. BPS has an estimated incidence of 0.15% to 1.7% and is the second most commonly identified developmental lung mass prenatally.2 BPS is approached similarly to the CPAM in terms of imaging and management.
The hallmark of BPS is a developmental mass fed by a systemic artery, usually arising from the thoracic aorta or upper abdominal aorta. A sequestration may be described as intralobar, if it shares the pleural investment with the normal lung, or extralobar, if it is isolated in its own pleural investment. The intralobar type usually also demonstrates pulmonary venous drainage, whereas the extralobar type usually has systemic venous drainage.15 It is important to be aware that an extralobar BPS may be located below the diaphragm and may mimic a suprarenal lesion such as adrenal hemorrhage or neuroblastoma.
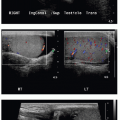
On prenatal imaging, a BPS will have variable echogenicity and degree of cystic components. The appearance is indistinguishable from a CPAM (Fig. 4.4). If a feeding artery arising from the aorta is identified, then the lesion may be considered a BPS or a hybrid lesion. As mentioned above in the discussion of a CPAM, prognostic features to document include large size, mediastinal shift, and hydrops. A careful assessment of the remaining fetal anatomy is imperative in these cases, because there is an association of other developmental anomalies in cases of extralobar sequestrations, including CDH, cardiac anomalies, and foregut duplication cysts.16
The postnatal management and imaging is the same as that for CPAM. Delivery of an infant with a prenatally diagnosed BPS, CPAM, or hybrid lesion at a tertiary care center for skilled neonatal medical attention is critical. Surgical resection will be timed according to the degree of respiratory distress, as described above. Presurgical imaging typically is limited to chest radiography and contrast-enhanced multidetector CT of the chest to evaluate extent of the lesion and to identify arterial supply and venous drainage (Fig. 4.4C-E).17
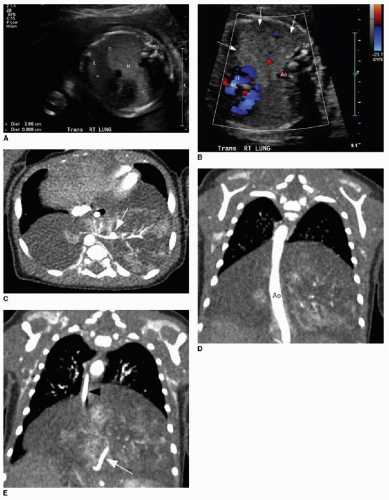 FIG. 4.4 • Bronchopulmonary sequestration seen on prenatal ultrasound and on postnatal CT angiogram. Sonographic axial image (A) through the fetal chest at gestational age 24 5/7 weeks shows an abnormal homogenously echogenic mass in the posterior lower chest crossing midline. Color Doppler (B) does not identify a feeding arterial vessel. Postnatal imaging at 3 days of life was performed using contrast-enhanced CT (C, axial; D,E, coronal). The congenital mass is bilateral, large, and predominantly solid. A feeding vessel (white arrows
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|