Scrotum
Teresa Chapman, MD
LEARNING OBJECTIVES
1. Differentiate typical testicular arterial waveform pattern in the infant versus adolescent.
2. Recognize normal appearance of the mediastinum testis.
3. List three sonographic findings indicative of torsion.
4. Indicate appropriate management for epididymitis in a young child.
5. Diagnose varicocele using ultrasound.
6. List three laboratory markers that are relevant to the diagnosis of a testicular mass.
7. Describe how findings of an intratesticular teratoma differ from that of an epidermoid cyst.
INTRODUCTION
Diagnostic imaging of the pediatric scrotum is performed using ultrasound, as in the adult. Indications for imaging in the neonate and infant include acute changes in the appearance of the scrotum, such as swelling and redness, as well as a palpable mass. In the older child and adolescent, abnormalities of the scrotum may present with acute onset of pain. The primary clinical differential diagnosis for a neonate or infant with an acute scrotum includes inguinal hernia, testicular torsion, and hydrocele. In contrast, the diagnostic considerations in an older child include testicular torsion, appendage torsion of the testis or epididymis, or epididymitis/orchitis. Painless palpable masses presenting at any age raise the possibilities of hydrocele, testicular neoplasm, paratesticular neoplasm, and varicocele. In this chapter, the essentials of scrotal ultrasound in a child will be discussed, as well as a description of acute abnormalities and neoplasms that may occur in the scrotum.
NORMAL ANATOMY
Ultrasound of the scrotum is performed using a high-resolution linear transducer. Axial and longitudinal views of the testis should be documented, with size measurements for volume calculation.1,2 The epididymis is also documented. It is important to assess the spermatic cord from the inguinal canal to the superior testis. Any intrascrotal abnormality should be evaluated with both gray-scale and color Doppler techniques.
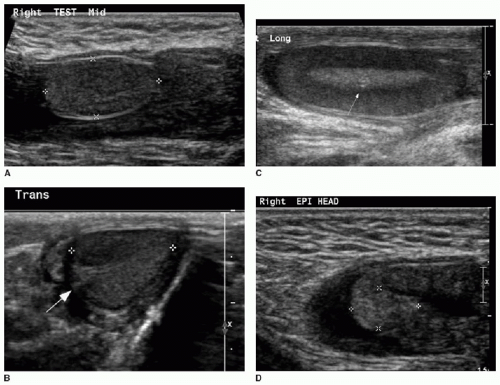
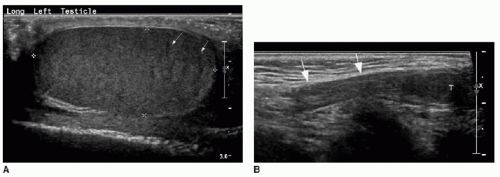
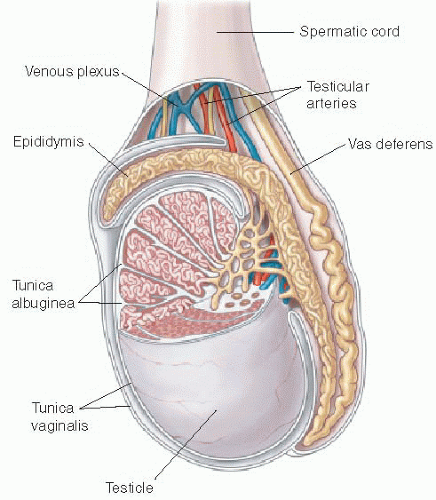
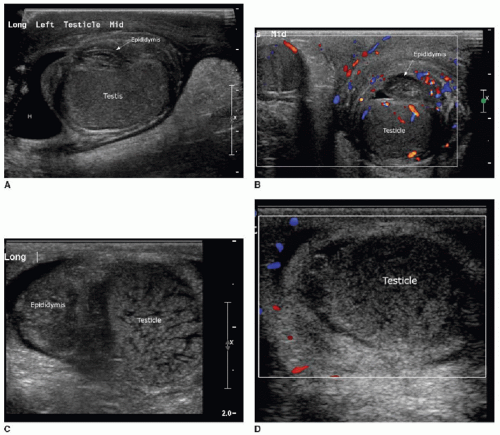
Both the shape and the echogenicity of the testis depend on the age of the individual being imaged. In the fetus and infant, the testis assumes a round morphology and later becomes oblong during puberty. Early in infancy, a transient rise in testosterone leads to an increase in the volume of the testis, and this gradually reduces and stabilizes by approximately 9 months of age. The testis will remain small throughout childhood and gradually enlarge through the stages of puberty.3,4 During most of the first decade, the testis is homogenously low in echogenicity (Fig. 24.1). At approximately 8 to 9 years of age, the echogenicity gradually increases until puberty, when the testis assumes a homogeneous intermediate echogenicity.5 It is normal to occasionally see hypoechoic linear striations through the parenchyma, which reflect interlobular septations between the organized lobules of seminiferous tubules (Fig. 24.2).1,2 Another normal structure identified sonographically is the echogenic mediastinum testis, which is a longitudinally oriented fibrous structure within the peripheral aspect of the testicular parenchyma. This is in continuity with the fibrous tunica albuginea surrounding the testicle. Just outside of that layer is the tunica vaginalis, which is a remnant of the peritoneal lining that extends through the inguinal canal early in development (Fig. 24.3).6
At the superior pole of the testis, one might visualize the testicular appendage, a developmental remnant of the müllerian duct. The appendage is usually less than 5 mm, round, and mildly echogenic.7 The epididymis is located along the posteromedial aspect of the testicle. This is an elongated structure that contains the seminiferous tubules. In the infant and child, it is typical to only see the epididymal head at the superomedial aspect of the testis (Fig. 24.1). The body and tail become more apparent with age, and the tail tapers distally to become the vas deferens. The testicular artery, pampiniform venous plexus, lymphatics, nerves, and the vas deferens travel through the spermatic cord (Fig. 24.2).6
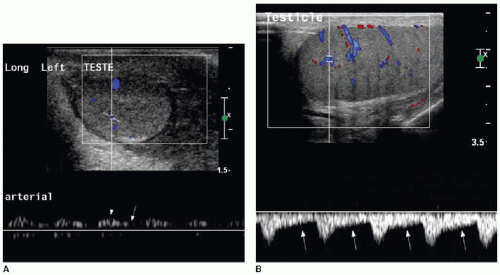
The testicular artery, which is the main blood supply to the testis, arborizes into penetrating branches at the mediastinum testis (see Fig. 24.3). Spectral wave analysis of the intratesticular arteries will demonstrate differences in the normal spectral waveform depending on age.8,9 In the young child, the arterial waveform shows a high resistance, whereas in the peripubertal and postpubertal boy, the testicular waveform has a lower resistance with readily appreciable forward diastolic flow (Fig. 24.4).
TESTICULAR TORSION
Abnormal twisting of the spermatic cord and testicle within the scrotal sac deprives the testicle of its blood supply, leading to testicular ischemia. This is an emergency that may merit imaging to confirm the diagnosis prior to surgical treatment.1,2,10 Testicular torsion presents with acute onset of pain, possibly accompanied by vomiting, absence of the cremasteric reflex, and abnormal elevation and horizontal position of the torsed testicle.11, 12 and 13 If the clinical presentation is fully consistent with testicular torsion, it is advantageous to pursue surgery immediately without diagnostic imaging to reduce the time of testicular ischemia. However, if the diagnosis is unclear, then ultrasound is appropriate. Imaging in the neonate, in particular, can reassure clinicians and parents that assuming the risks of anesthesia and surgery are validated.14,15 Neonatal testicular torsion most commonly occurs prior to birth. A remote torsion may result in testicular ischemia and volume loss that is so advanced, the abnormality is apparent by the palpation of a firm nontender testis associated with a discolored scrotum, or by recognition of unilateral anorchia.12,14, 15 and 16 Acute torsion in the neonate, however, does occur, and the accompanying signs of scrotal enlargement, redness, and pain may be variably present.
The underlying pathogenesis of torsion differs in the neonatal-infantile period from that in the older child. Testicular torsion is usually extravaginal in the fetus and infant, meaning the entire testis and tunica vaginalis twists abnormally with the spermatic cord within the scrotum.1,2,11,16 This phenomenon tends to
be unilateral. In contrast, the older child usually presents with intravaginal torsion (also called the “bell-clapper deformity”), which describes twisting of the spermatic cord and testis within the tunica vaginalis layer. The bell-clapper deformity is usually bilateral, and therefore, fixation of the asymptomatic side to prevent future torsion is pursued by suturing the testicle within the scrotum (orchidopexy) at the time of surgical detorsion, as well as orchidopexy for the affected side.1,2,5,12
be unilateral. In contrast, the older child usually presents with intravaginal torsion (also called the “bell-clapper deformity”), which describes twisting of the spermatic cord and testis within the tunica vaginalis layer. The bell-clapper deformity is usually bilateral, and therefore, fixation of the asymptomatic side to prevent future torsion is pursued by suturing the testicle within the scrotum (orchidopexy) at the time of surgical detorsion, as well as orchidopexy for the affected side.1,2,5,12
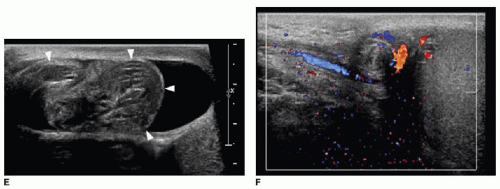
Gray-scale appearance of a torsed testis depends on the length of ischemia. Testicular enlargement and edema, evidenced by an exaggerated striated appearance, may indicate a lower likelihood of viability with detorsion.14,17 Preservation of normal echogenicity indicates the testis is viable (Fig. 24.5A).18 The necrotic testis will appear markedly heterogenous in its echogenicity (Fig. 24.5B). Ultrasound should also evaluate the appearance of the spermatic cord, which will appear as a boggy “pseudomass”
if it is torsed (Fig. 24.5E).19,20 The clinical signs and symptoms of torsion and the gray-scale appearance of the testicular parenchyma are important in the diagnosis and in determining if rapid detorsion is a viable option.21
if it is torsed (Fig. 24.5E).19,20 The clinical signs and symptoms of torsion and the gray-scale appearance of the testicular parenchyma are important in the diagnosis and in determining if rapid detorsion is a viable option.21
Doppler evaluation of the testis also contributes to making the diagnosis of torsion. Absent arterial inflow and venous outflow unilaterally confirms the diagnosis (Fig. 24.5).2,6,9,10,12,22 However, just as the Doppler evaluation can be confusing in the context of ovarian torsion in the female (discussed in Chapter 22), Doppler can be misleading in the context of testicular torsion. In a study of 41 testicular torsion cases,22 10 individuals had normal blood flow to the torsed testicle. The key to diagnosis in 8 of those 10 cases was observation of twisting of the spermatic cord (Fig. 24.5E,F). Partial torsion may manifest as relatively lower vascularity to the symptomatic side compared with the normal side using color and power Doppler.5 Spontaneous detorsion may occur, and if so, exaggerated blood flow to the symptomatic side may be evident using Doppler. In general, hyperemia to the symptomatic side would imply an inflammatory nonsurgical condition. However, if spontaneous detorsion results in unilateral hyperemia, the presenting symptoms such as pain should immediately dissipate, allowing for distinction between spontaneous detorsion and an inflammatory cause for hyperemia.1,4,9,12
Ultrasound is the primary mode of imaging of the scrotum. Nevertheless, scrotal scintigraphy has been used in the past and will be mentioned briefly here. Technetium-99m pertechnetate injected intravenously can be used as a blood flow marker. Pinhole planar images of the scrotum acquired immediately after radiotracer injection show a region of photopenia in the location of a torsed testis. The ipsilateral scrotal sac may take up an asymmetric amount of radiotracer, producing a circular rim around the photopenic ischemic testicle. The scintigraphic evaluation was reported to have a sensitivity and specificity of 90% and 89%, respectively, for the detection of torsion.23
Testicular torsion must be reversed for testicular viability. The longer the time of torsion, the lower the likelihood of salvage: while nearly all torsed testes operated upon within 6 hours of symptom onset can be saved, the salvage rate declines to 70% for symptoms present for 6 to 12 hours and 20% for 12 to 24 hours.24,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree