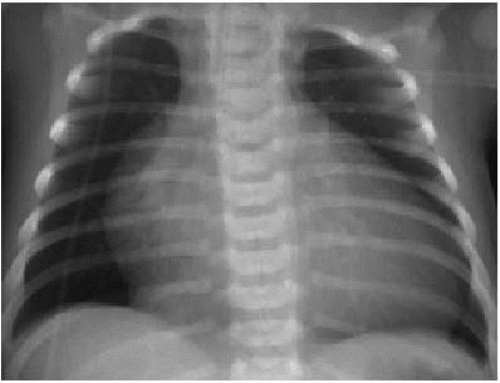
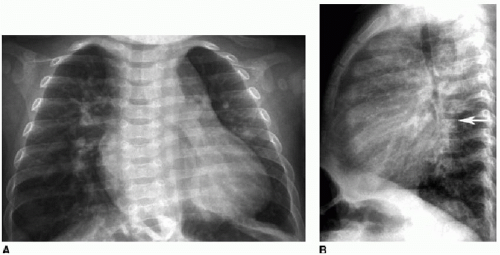
Is the patient cyanotic or acyanotic?
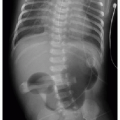
Is pulmonary vascularity increased or low/normal? The appearance of pulmonary arterial overcirculation, or “shunt vascularity,” was characterized in Chapter 9. Determining whether there is enlargement and abundance of pulmonary arteries takes experience. Identifying low pulmonary vascularity is perhaps even more challenging (Fig. 10.1). Fortunately for the radiologist, in the cyanotic child, both low and normal pulmonary arterial flow are managed similarly in that they both exhibit pulmonary-systemic (right-to-left) shunting. Therefore, the question for radiography interpretation boils down to whether or not pulmonary vascularity is increased (Fig. 9.6).1,2
Is there cardiomegaly or a normal heart size?
over the thoracic spine (Fig. 9.7B). On echocardiography, CT, or MR, the left-to-right shunt may be directly visualized, and quantification of flow across the defect may be performed with MR or echo by calculating the ratio of flow through the main pulmonary artery (Qp) versus flow through the ascending aorta (Qs). Another method for predicting the type of shunt present is the age at presentation. VSDs and atrioventricular septal defects (AVSDs) present early in life, usually in infancy. ASDs typically present in preschool-aged children or in older children, even presenting as late as in adulthood (“A” in ASD for Adults). Patent ductus arteriosus (PDA) usually presents in premature neonates and makes up the majority of left-to-right shunts in these patients.4
Table 10.1 CLASSIFICATION OF SHUNT LESIONS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree